The Dietary Reference Intake (DRI) is a system of nutrition recommendations from the National Academy of Medicine (NAM)[a] of the National Academies (United States).[1] It was introduced in 1997 in order to broaden the existing guidelines known as Recommended Dietary Allowances(RDAs, see below). The DRI values differ from those used in nutrition labeling on food and dietary supplement products in the U.S. and Canada, which uses Reference Daily Intakes (RDIs) and Daily Values (%DV) which were based on outdated RDAs from 1968 but were updated as of 2016.[2]
Parameters[edit]
DRI provides several different types of reference values:[1]
- Estimated Average Requirements (EAR), expected to satisfy the needs of 50% of the people in that age group based on a review of the scientific literature.
- Recommended Dietary Allowances (RDA), the daily dietary intake level of a nutrient considered sufficient by the Food and Nutrition Board of the Institute of Medicine to meet the requirements of 97.5% of healthy individuals in each life-stage and sex group. The definition implies that the intake level would cause a harmful nutrient deficiency in just 2.5%. It is calculated based on the EAR and is usually approximately 20% higher than the EAR (See Calculating the RDA).
- Adequate Intake (AI), where no RDA has been established, but the amount established is somewhat less firmly believed to be adequate for everyone in the demographic group.
- Tolerable upper intake levels (UL), to caution against excessive intake of nutrients (like vitamin A) that can be harmful in large amounts. This is the highest level of sustained daily nutrient consumption that is considered to be safe for, and cause no side effects in, 97.5% of healthy individuals in each life-stage and sex group. The definition implies that the intake level would cause a harmful nutrient excess in just 2.5%. The European Food Safety Authority (EFSA) has also established ULs which do not always agree with U.S. ULs. For example, adult zinc UL is 40 mg in U.S. and 25 mg in EFSA.[3]
- Acceptable Macronutrient Distribution Ranges (AMDR), a range of intake specified as a percentage of total energy intake. Used for sources of energy, such as fats and carbohydrates.
DRIs are used by both the United States and Canada, and are intended for the general public and health professionals. Applications include:
- Composition of diets for schools, prisons, hospitals or nursing homes
- Industries developing new foods and dietary supplements
- Healthcare policy makers and public health officials
https://en.wikipedia.org/wiki/Dietary_Reference_Intake
External links[edit]
- Dietary Reference Intakes at United States National Agricultural Library
- Current USA dietary guidelines 2020–2025
- FDA
- USDA
- Laboratory, Research Facility, Evidence compilation, Test Site, Researcher, etc..
Reference intakes (RIs) are a means of communicating maximum recommended nutrient intake to the public. Reference Intakes replaced the term Guideline Daily Amount (GDA), although the principles behind both are the same. The major difference is that GDAs existed for men, women and children; there is only one set of RIs for an average adult.[1]
| Energy or nutrient | Reference Intake |
|---|---|
| Energy | 8400 kJ / 2000 kcal |
| Total fat | 70 g |
| Saturates | 20 g |
| Carbohydrates | 260 g |
| Sugars | 90 g |
| Protein | 50 g |
| Salt | 6 g |
These RIs are based on the requirements for an average woman with no special dietary requirements and assume an energy intake of 8400 kJ.[1] The information is for guidance only and should not be considered individual advice.[1]
The change from GDA to RI on labels on pre-packaged food and drinks sold in the UK is due to Regulation (EU) 1169/2011.[2][3] The intention of the EU Regulation is to harmonise across Europe the content, expression and presentation of the nutrition information given to consumers.
Since RIs are for an average adult, concerns have been raised by major retailers and manufacturers that they may face criticism for misrepresenting the contribution to the diet of products targeted at children, particularly given concerns around children's diet and obesity levels.[4]
RIs can be combined with traffic light labeling to make the information easily and rapidly understood.[5]
https://en.wikipedia.org/wiki/Reference_intake
Body mass index (BMI) is a value derived from the mass (weight) and height of a person. The BMI is defined as the body mass divided by the square of the body height, and is expressed in units of kg/m2, resulting from mass in kilograms and height in metres.
The BMI may be determined using a table[a] or chart which displays BMI as a function of mass and height using contour lines or colours for different BMI categories, and which may use other units of measurement (converted to metric units for the calculation).[b]
The BMI is a convenient rule of thumb used to broadly categorize a person as underweight, normal weight, overweight, or obese based on tissue mass (muscle, fat, and bone) and height. Major adult BMI classifications are underweight (under 18.5 kg/m2), normal weight (18.5 to 24.9), overweight (25 to 29.9), and obese (30 or more).[1] When used to predict an individual's health, rather than as a statistical measurement for groups, the BMI has limitations that can make it less useful than some of the alternatives, especially when applied to individuals with abdominal obesity, short stature, or unusually high muscle mass.
BMIs under 20 and over 25 have been associated with higher all-causes mortality, with the risk increasing with distance from the 20–25 range.[2] However, the ideal range varies by race, with a BMI that is considered normal for a group of Europeans being unhealthily high for a group of Asians.
https://en.wikipedia.org/wiki/Body_mass_index
A notebook (also known as a notepad, writing pad, drawing pad, or legal pad) is a book or stack of paper pages that are often ruled and used for purposes such as recording notes or memoranda, other writing, drawing or scrapbooking.[citation needed]
https://en.wikipedia.org/wiki/Notebook
An electronic calculator is typically a portable electronic device used to perform calculations, ranging from basic arithmetic to complex mathematics.
https://en.wikipedia.org/wiki/Calculator
A pen is a common writing instrument that applies ink to a surface, usually paper, for writing or drawing.[1]
https://en.wikipedia.org/wiki/Pen
The Schofield Equation is a method of estimating the basal metabolic rate (BMR) of adult men and women published in 1985.[1]
This is the equation used by the WHO in their technical report series.[2] The equation that is recommended to estimate BMR by the US Academy of Nutrition and Dietetics is the Mifflin-St. Jeor equation.[3]
The equations for estimating BMR in kJ/day (kilojoules per day) from body mass (kg) are:[4]
Men:
| Age | Equation (kJ/day) | SEE |
|---|---|---|
| < 3 | 249 × W - 127 | 292 |
| 3–10 | 95 × W + 2110 | 280 |
| 10–18 | 74 × W + 2754 | 441 |
| 18–30 | 63 × W + 2896 | 641 |
| 30–60 | 48 × W + 3653 | 700 |
| > 60 | 49 × W + 2459 | 686 |
Women:
| Age | Equation (kJ/day) | SEE |
|---|---|---|
| < 3 | 244 × W - 130 | 246 |
| 3–10 | 85 × W + 2033 | 292 |
| 10–18 | 56 × W + 2898 | 466 |
| 18–30 | 62 × W + 2036 | 497 |
| 30–60 | 34 × W + 3538 | 465 |
| > 60 | 38 × W + 2755 | 451 |
The equations for estimating BMR in kcal/day (kilocalories per day) from body mass (kg) are:
Men:
| Age | Equation (kcal/day) | SEE |
|---|---|---|
| < 3 | 59.512 × W - 30.4 | 70 |
| 3–10 | 22.706 × W + 504.3 | 67 |
| 10–18 | 17.686 × W + 658.2 | 105 |
| 18–30 | 15.057 × W + 692.2 | 153 |
| 30–60 | 11.472 × W + 873.1 | 167 |
| > 60 | 11.711 × W + 587.7 | 164 |
Women:
| Age | Equation (kcal/day) | SEE |
|---|---|---|
| < 3 | 58.317 × W - 31.1 | 59 |
| 3–10 | 20.315 × W + 485.9 | 70 |
| 10–18 | 13.384 × W + 692.6 | 111 |
| 18–30 | 14.818 × W + 486.6 | 119 |
| 30–60 | 8.126 × W + 845.6 | 111 |
| > 60 | 9.082 × W + 658.5 | 108 |
Key:
W = Body weight in kilograms
SEE = Standard error of estimation
The raw figure obtained by the equation should be adjusted up or downwards, within the confidence limit suggested by the quoted estimation errors, and according to the following principles:
Subjects leaner and more muscular than usual require more energy than the average. Obese subjects require less. Patients at the young end of the age range for a given equation require more energy. Patients at the high end of the age range for a given equation require less energy.
Effects of age and body mass may cancel out: an obese 30-year-old or an athletic 60-year-old may need no adjustment from the raw figure.
To find actual energy needed per day (Estimated Energy Requirement), the base metabolism must then be multiplied by an activity factor. These are as follows:
- Sedentary people of both genders should multiply by 1.3. Sedentary is very physically inactive, inactive in both work and leisure.
- Lightly active men should multiply by 1.6 and women by 1.5. Lightly active means the daily routine includes some walking, or intense exercise once or twice per week. Most students are in this category.
- Moderately active men should multiply by 1.7 and women by 1.6. Moderately active means intense exercise lasting 20–45 minutes at least three time per week, or a job with a lot of walking, or a moderate intensity job.
- Very Active men should multiply by 2.1 and women by 1.9. Very active means intense exercise lasting at least an hour per day, or a heavy physical job, such as a mail carrier or an athlete in training.
- Extremely active men should multiply by 2.4 and women by 2.2. Extremely active means an athlete on an unstoppable training schedule or a very demanding job, such as working in the armed forces or shoveling coal.
These equations were published in 1989 in the dietary guidelines and formed the RDA's for a number of years. The activity factor used by the USDAwas 1.6. In the UK, a lower activity factor of 1.4 is used. The equation has now been replaced by the Institute of Medicine Equation in September 2002 in the US, however is still currently used by the FAO/WHO/UNU.
https://en.wikipedia.org/wiki/Schofield_equation
Basal metabolic rate (BMR) is the rate of energy expenditure per unit time by endothermic animals at rest.[1] It is reported in energy units per unit time ranging from watt (joule/second) to ml O2/min or joule per hour per kg body mass J/(h·kg). Proper measurement requires a strict set of criteria be met. These criteria include being in a physically and psychologically undisturbed state, in a thermally neutral environment, while in the post-absorptive state(i.e., not actively digesting food).[1] In bradymetabolic animals, such as fish and reptiles, the equivalent term standard metabolic rate (SMR) is used. It follows the same criteria as BMR, but requires the documentation of the temperature at which the metabolic rate was measured. This makes BMR a variant of standard metabolic rate measurement that excludes the temperature data, a practice that has led to problems in defining "standard" rates of metabolism for many mammals.[1]
Metabolism comprises the processes that the body needs to function.[2] Basal metabolic rate is the amount of energy per unit of time that a person needs to keep the body functioning at rest. Some of those processes are breathing, blood circulation, controlling body temperature, cell growth, brain and nerve function, and contraction of muscles. Basal metabolic rate affects the rate that a person burns calories and ultimately whether that individual maintains, gains, or loses weight. The basal metabolic rate accounts for about 60 to 75% of the daily calorie expenditure by individuals. It is influenced by several factors. In humans, BMR typically declines by 1–2% per decade after age 20, mostly due to loss of fat-free mass,[3] although the variability between individuals is high.[4]
https://en.wikipedia.org/wiki/Basal_metabolic_rate
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, radiation, and physical properties of matter. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopicconstituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology.
Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Nicolas Léonard Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars.[1]Scots-Irish physicist Lord Kelvin was the first to formulate a concise definition of thermodynamics in 1854[2] which stated, "Thermo-dynamics is the subject of the relation of heat to forces acting between contiguous parts of bodies, and the relation of heat to electrical agency."
The initial application of thermodynamics to mechanical heat engines was quickly extended to the study of chemical compounds and chemical reactions. Chemical thermodynamics studies the nature of the role of entropy in the process of chemical reactions and has provided the bulk of expansion and knowledge of the field.[3][4][5][6][7][8][9][10][11] Other formulations of thermodynamics emerged. Statistical thermodynamics, or statistical mechanics, concerns itself with statisticalpredictions of the collective motion of particles from their microscopic behavior. In 1909, Constantin Carathéodory presented a purely mathematical approach in an axiomatic formulation, a description often referred to as geometrical thermodynamics.
https://en.wikipedia.org/wiki/Thermodynamics
Bomb calorimeters[edit]
A bomb calorimeter is a type of constant-volume calorimeter used in measuring the heat of combustion of a particular reaction. Bomb calorimeters have to withstand the large pressure within the calorimeter as the reaction is being measured. Electrical energy is used to ignite the fuel; as the fuel is burning, it will heat up the surrounding air, which expands and escapes through a tube that leads the air out of the calorimeter. When the air is escaping through the copper tube it will also heat up the water outside the tube. The change in temperature of the water allows for calculating calorie content of the fuel.
In more recent calorimeter designs, the whole bomb, pressurized with excess pure oxygen (typically at 30 atm) and containing a weighed mass of a sample (typically 1–1.5 g) and a small fixed amount of water (to saturate the internal atmosphere, thus ensuring that all water produced is liquid, and removing the need to include enthalpy of vaporization in calculations), is submerged under a known volume of water (ca. 2000 ml) before the charge is electrically ignited. The bomb, with the known mass of the sample and oxygen, form a closed system — no gases escape during the reaction. The weighed reactant put inside the steel container is then ignited. Energy is released by the combustion and heat flow from this crosses the stainless steel wall, thus raising the temperature of the steel bomb, its contents, and the surrounding water jacket. The temperature change in the water is then accurately measured with a thermometer. This reading, along with a bomb factor (which is dependent on the heat capacity of the metal bomb parts), is used to calculate the energy given out by the sample burn. A small correction is made to account for the electrical energy input, the burning fuse, and acid production (by titration of the residual liquid). After the temperature rise has been measured, the excess pressure in the bomb is released.
Basically, a bomb calorimeter consists of a small cup to contain the sample, oxygen, a stainless steel bomb, water, a stirrer, a thermometer, the dewar or insulating container (to prevent heat flow from the calorimeter to the surroundings) and ignition circuit connected to the bomb. By using stainless steel for the bomb, the reaction will occur with no volume change observed.
Since there is no heat exchange between the calorimeter and surroundings (Q = 0) (adiabatic), no work is performed (W = 0)
Thus, the total internal energy change
Also, total internal energy change
- (constant volume )
where is heat capacity of the bomb
Before the bomb can be used to determine heat of combustion of any compound, it must be calibrated. The value of can be estimated by
- and can be measured;
In the laboratory, is determined by running a compound with known heat of combustion value:
Common compounds are benzoic acid () or p-methyl benzoic acid ().
Temperature (T) is recorded every minute and
A small factor contributes to the correction of the total heat of combustion is the fuse wire. Nickel fuse wire is often used and has heat of combustion: 981.2 cal/g.
In order to calibrate the bomb, a small amount (~ 1 g) of benzoic acid, or p-methyl benzoic acid is weighed. A length of nickel fuse wire (~10 cm) is weighed both before and after the combustion process. Mass of fuse wire burned
The combustion of sample (benzoic acid) inside the bomb
Once value of the bomb is determined, the bomb is ready to use to calculate heat of combustion of any compounds by
Combustion of non-flammables[edit]
The higher pressure and concentration of O
2 in the bomb system can render combustible some compounds that are not normally flammable. Some substances do not combust completely, making the calculations harder as the remaining mass has to be taken into consideration, making the possible error considerably larger and compromising the data.
When working with compounds that are not as flammable (that might not combust completely) one solution would be to mix the compound with some flammable compounds with a known heat of combustion and make a pallet with the mixture. Once the of the bomb is known, the heat of combustion of the flammable compound (CFC), of the wire (CW) and the masses (mFC and mW), and the temperature change (ΔT), the heat of combustion of the less flammable compound (CLFC) can be calculated with:
- CLFC = Cv ΔT − CFC mFC − CW mW[6][failed verification]
https://en.wikipedia.org/wiki/Calorimeter#Bomb_calorimeters
The calorie is a unit of energy defined as the amount of heat needed to raise the temperature of a quantity of water by one degree.
For historical reasons, two main definitions of calorie are in wide use. The small calorie or gram calorie(usually denoted cal) is the amount of heat needed to raise the temperature of one gram of water by one degree Celsius (or one kelvin).[1][2] The large calorie, food calorie, or kilocalorie (Cal, Calorie or kcal), most widely used in nutrition,[3] is the amount of heat needed to cause the same increase in one kilogramof water.[4] Thus, 1 kilocalorie (kcal) = 1000 calories (cal). By convention in food science, the large calorie is commonly called Calorie (with a capital C by some authors to distinguish from the smaller unit).[5] In most countries, labels of industrialized food products are required to indicate the nutritional energy value in (kilo or large) calories per serving or per weight.
Calorie relates directly to the metric system, and therefore to the SI system. It has been regarded as obsolete within the scientific community since the adoption of the SI system, but is still in some use.[3] The SI unit of energy is the joule. One calorie is defined as exactly 4.184 J, and one Calorie (kilocalorie) is 4184 J.
https://en.wikipedia.org/wiki/Calorie
The calorie is a unit of energy defined as the amount of heat needed to raise the temperature of a quantity of water by one degree.
For historical reasons, two main definitions of calorie are in wide use. The small calorie or gram calorie(usually denoted cal) is the amount of heat needed to raise the temperature of one gram of water by one degree Celsius (or one kelvin).[1][2] The large calorie, food calorie, or kilocalorie (Cal, Calorie or kcal), most widely used in nutrition,[3] is the amount of heat needed to cause the same increase in one kilogramof water.[4] Thus, 1 kilocalorie (kcal) = 1000 calories (cal). By convention in food science, the large calorie is commonly called Calorie (with a capital C by some authors to distinguish from the smaller unit).[5] In most countries, labels of industrialized food products are required to indicate the nutritional energy value in (kilo or large) calories per serving or per weight.
Calorie relates directly to the metric system, and therefore to the SI system. It has been regarded as obsolete within the scientific community since the adoption of the SI system, but is still in some use.[3] The SI unit of energy is the joule. One calorie is defined as exactly 4.184 J, and one Calorie (kilocalorie) is 4184 J.
History[edit]
The calorie was first introduced by Nicolas Clément, as a unit of heat energy, in lectures during the years 1819–1824. This was the "large" calorie, viz. modern kilocalorie.[3][6] The term entered French and English dictionaries between 1841 and 1867. It comes from Latin calor 'heat'.
The "small" calorie (modern calorie) was introduced by Pierre Antoine Favre (Chemist) and Johann T. Silbermann (Physicist) in 1852. In 1879, Marcellin Berthelot distinguished between gram-calorie (modern calorie) and kilogram-calorie (modern kilocalorie).[6] Berthelot also introduced the convention of capitalizing the kilogram-calorie, as Calorie.
The use of the kilogram-calorie (kcal) for nutrition was introduced to the American public by Wilbur Olin Atwater, a professor at Wesleyan University, in 1887.[3]
The modern calorie (cal) was first recognized as a unit of the cm-g-s system (cgs) in 1896,[6] alongside the already-existing cgs unit of energy, the erg(first suggested by Clausius in 1864, under the name ergon, and officially adopted in 1882).
Already in 1928 there were serious complaints about the possible confusion arising from the two main definitions of the calorie and whether the notion of using the capital letter to distinguish them was sound.[7] Use of the calorie was officially deprecated by the ninth General Conference on Weights and Measures, in 1948.[8]
The alternate spelling calory is archaic.
Definitions[edit]
The modern (small) calorie is defined as the amount of energy needed to increase the temperature of 1 gram of water by 1 °C (or 1 K, which is the same increment).[1][2] The definition depends on the atmospheric pressure and the starting temperature. Accordingly, several different precise definitions of the calorie have been used.
| Name | Symbol | Conversions | Definition and notes |
|---|---|---|---|
| Thermochemicalcalorie | calth | ≡ 4.184 J | The amount of energy equal to exactly 4.184 J (Joules) and 1 kJ = 0.239 kcal.[9][10][11] (a). |
| 4 °C calorie | cal4 | ≈ 4.204 J ≈ 0.003985 BTU≈ 1.168×10−6 kW⋅h ≈ 2.624×1019 eV | The amount of energy required to warm one gram of air-free water from 3.5 to 4.5 °C at standard atmospheric pressure. (c) |
| 15 °C calorie | cal15 | ≈ 4.1855 J ≈ 0.0039671 BTU≈ 1.1626×10−6 kW⋅h ≈ 2.6124×1019 eV | The amount of energy required to warm one gram of air-free water from 14.5 to 15.5 °C at standard atmospheric pressure. (c) Experimental values of this calorie ranged from 4.1852 to 4.1858 J. The CIPM in 1950 published a mean experimental value of 4.1855 J, noting an uncertainty of 0.0005 J.[9] |
| 20 °C calorie | cal20 | ≈ 4.182 J ≈ 0.003964 BTU≈ 1.162×10−6 kW⋅h ≈ 2.610×1019 eV | The amount of energy required to warm one gram of air-free water from 19.5 to 20.5 °C at standard atmospheric pressure. (c) |
| Mean calorie | calmean | ≈ 4.190 J ≈ 0.003971 BTU≈ 1.164×10−6 kW⋅h ≈ 2.615×1019 eV | Defined as 1⁄100 of the amount of energy required to warm one gram of air-free water from 0 to 100 °C at standard atmospheric pressure. (c) |
| International Steam Tablecalorie (1929) | ≈ 4.1868 J ≈ 0.0039683 BTU≈ 1.1630×10−6 kW⋅h ≈ 2.6132×1019 eV | Defined as 1⁄860 "international" watt hours = 180⁄43 "international" joules exactly. (b) | |
| International Steam Table calorie (1956) | calIT | ≡ 4.1868 J ≈ 0.0039683 BTU= 1.1630×10−6 kW⋅h ≈ 2.6132×1019 eV | Defined as 1.163 mW⋅h = 4.1868 J exactly. This definition was adopted by the Fifth International Conference on Properties of Steam (London, July 1956).[9] |
- (a) The 'Thermochemical calorie' was defined by Rossini simply as 4.1833 international joules in order to avoid the difficulties associated with uncertainties about the heat capacity of water. It was later redefined as 4.1840 J exactly.[12]
- (b) The figure depends on the conversion factor between "international joules" and "absolute" (modern, SI) joules. Using the mean international ohm and volt (1.00049 Ω, 1.00034 V[13]), the "international joule" is about 1.00019 J, using the US international ohm and volt (1.000495 Ω, 1.000330 V) it is about 1.000165 J, giving 4.18684 and 4.18674 J, respectively.
- (c) The standard atmospheric pressure can be taken to be 101.325 kPa.
The two definitions most common in older literature appear to be the 15 °C calorie and the thermochemical calorie. Until 1948, the latter was defined as 4.1833 international joules; the current standard of 4.184 J was chosen to have the new thermochemical calorie represent the same quantity of energy as before.[10]
The calorie was first defined specifically to measure energy in the form of heat, especially in experimental calorimetry.[14]
Nutrition[edit]
In a nutritional context, the kilojoule (kJ) is the SI unit of food energy, although the calorie is commonly used.[15][16] The word calorie is commonly used with the number of kilocalories (kcal) of nutritional energy measured.
In the United States, most nutritionists prefer the unit kilocalorie to the unit kilojoules, whereas most physiologists prefer to use kilojoules. In the majority of other countries, nutritionists prefer the kilojoule to the kilocalorie.[17] US food labelling laws require the use of kilocalories (under the name of "Calories"); kilojoules are permitted to be included on food labels alongside kilocalories, but most food labels do not do so. In Australia, kilojoules are officially preferred over kilocalories, but kilocalories retain some degree of popular use.[18] Australian and New Zealand food labelling laws require the use of kilojoules; kilocalories are allowed to be included on labels in addition to kilojoules, but are not required.[19] EU food labelling laws require both kilojoules and kilocalories on all nutritional labels, with the kilojoules listed first.[20]
To facilitate comparison, specific energy or energy density figures are often quoted as "calories per serving" or "kcal per 100 g". A nutritional requirement or consumption is often expressed in calories or kilocalories per day.
Food nutrients as fat (lipids) contains 9 kilocalories per gram (kcal/g), while carbohydrate (sugar) or protein contains approximately 4 kcal/g.[21] Alcohol in food contains 7 kcal/g.[22] Food nutrients are also often quoted "per 100 g".
Chemistry[edit]
In other scientific contexts, the term calorie almost always refers to the small calorie. Even though it is not an SI unit, it is still used in chemistry. For example, the energy released in a chemical reaction per mole of reagent is occasionally expressed in kilocalories per mole.[23] Typically, this use was largely due to the ease with which it could be calculated in laboratory reactions, especially in aqueous solution: a volume of reagent dissolved in water forming a solution, with concentration expressed in moles per litre (1 litre weighing 1 kilogram), will induce a temperature change in degrees Celsius in the total volume of water solvent, and these quantities (volume, molar concentration and temperature change) can then be used to calculate energy per mole. It is also occasionally used to specify energy quantities that relate to reaction energy, such as enthalpy of formation and the size of activation barriers.[24] However, its use is being superseded by the SI unit, the joule, and multiples thereof such as the kilojoule.
Measurement of energy content of food[edit]
In the past, a bomb calorimeter was used to determine the energy content of food by burning a sample and measuring a temperature change in the surrounding water. Today, this method is not commonly used in the United States and has been replaced by calculating the energy content indirectly from adding up the energy provided by energy-containing nutrients of food (such as protein, carbohydrates, and fats), the Modified Atwater system. The fibre content is also subtracted to account for the fact that fibre is not digested by the body.[21]
See also[edit]
https://en.wikipedia.org/wiki/Calorie
Energy is defined via work, so the SI unit of energy is the same as the unit of work – the joule (J), named in honour of James Prescott Joule and his experiments on the mechanical equivalent of heat. In slightly more fundamental terms, 1 joule is equal to 1 newton metre and, in terms of SI base units
An energy unit that is used in atomic physics, particle physics and high energy physics is the electronvolt (eV). One eV is equivalent to 1.60217653×10−19 J.
In spectroscopy the unit cm−1 ≈ 0.0001239842 eV is used to represent energy since energy is inversely proportional to wavelength from the equation .
In discussions of energy production and consumption, the units barrel of oil equivalent and ton of oil equivalent are often used.
https://en.wikipedia.org/wiki/Units_of_energy
https://en.wikipedia.org/wiki/energy
In thermodynamics, heat is energy in transfer to or from a thermodynamic system, by mechanisms other than thermodynamic work or transfer of matter.[1][2][3][4][5][6][7] The various mechanisms of energy transfer that define heat are stated in the next section of this article.
Like thermodynamic work, heat transfer is a process involving more than one system, not a property of any one system. In thermodynamics, energy transferred as heat contributes to change in the system's cardinal energy variable of state, for example its internal energy, or for example its enthalpy. This is to be distinguished from the ordinary language conception of heat as a property of an isolated system.
The quantity of energy transferred as heat in a process is the amount of transferred energy excluding any thermodynamic work that was done and any energy contained in matter transferred. For the precise definition of heat, it is necessary that it occur by a path that does not include transfer of matter.[8]
Though not immediately by the definition, but in special kinds of process, quantity of energy transferred as heat can be measured by its effect on the states of interacting bodies. For example, respectively in special circumstances, heat transfer can be measured by the amount of ice melted, or by change in temperatureof a body in the surroundings of the system.[9] Such methods are called calorimetry.
The conventional symbol used to represent the amount of heat transferred in a thermodynamic process is Q. As an amount of energy (being transferred), the SI unit of heat is the joule (J).
https://en.wikipedia.org/wiki/Heat
Food energy is chemical energy that animals (including humans) derive from their food and molecular oxygen[1] through the process of cellular respiration. Cellular respiration involves either the process of joining oxygen from air with the molecules of food (aerobic respiration) or the process of reorganizing the atoms within the molecules (anaerobic respiration).
Humans and other animals need a minimum intake of food energy to sustain their metabolism and to drive their muscles. Foods are composed chiefly of carbohydrates, fats, proteins, water, vitamins, and minerals. Carbohydrates, fats, proteins, and water represent virtually all the weight of food, with vitamins and minerals making up only a small percentage of the weight. (Carbohydrates, fats, and proteins comprise ninety percent of the dry weight of foods.[2]) Organisms derive food energy from carbohydrates, fats and proteins as well as from organic acids, polyols, and ethanol present in the diet.[3]Some diet components that provide little or no food energy, such as water, minerals, vitamins, cholesterol, and fiber, may still be necessary to health and survival for other reasons. Water, minerals, vitamins, and cholesterol are not broken down (they are used by the body in the form in which they are absorbed) and so cannot be used for energy. Fiber, a type of carbohydrate, cannot be completely digested by the human body. Ruminants can extract food energy from the respiration of cellulose because of bacteria in their rumens.
Using the International System of Units, researchers measure energy in joules (J) or in its multiples; the kilojoule (kJ) is most often used for food-related quantities. An older metric system unit of energy, still widely used in food-related contexts, is the calorie; more precisely, the "food calorie", "large calorie" or kilocalorie (kcal or Cal), equal to 4.184 kilojoules.[a] Within the European Union, both the kilocalorie ("kcal") and kilojoule ("kJ") appear on nutrition labels. In many countries, only one of the units is displayed; in the US and Canada labels spell out the unit as "calorie" or as "Calorie".
Fats and ethanol have the greatest amount of food energy per mass, 37 and 29 kJ/g (9 and 7 kcal/g), respectively. Proteins and most carbohydrates have about 17 kJ/g (4 kcal/g). The differing energy density of foods (fat, alcohols, carbohydrates and proteins) lies mainly in their varying proportions of carbon, hydrogen, and oxygen atoms: For food of elemental composition CcHhOoNn, the heat of combustion underlying the food energy is given by the empirical formula
to a good approximation (±3%).[1] Carbohydrates that are not easily absorbed, such as fiber, or lactose in lactose-intolerant individuals, contribute less food energy. Polyols (including sugar alcohols) and organic acids contribute 10 and 13 kJ/g (2 and 3 kcal/g) respectively.[4] The amount of water, fat, and fiber in foods determines those foods' energy density. Theoretically, one could measure food energy in different ways, using (say) the Gibbs free energy of combustion, or the amount of ATP generated by metabolizing the food. However, the convention is to use the heat of the oxidation reaction, with the water substance produced being in the liquid phase. Conventional food energy is based on heats of combustion in a bomb calorimeter and corrections that take into consideration the efficiency of digestion and absorption and the production of urea and other substances in the urine. The American chemist Wilbur Atwater worked these corrections out in the late 19th century.[5] (See Atwater system for more detail.)
Each food item has a specific metabolizable energy intake (MEI). This value can be approximated by multiplying the total amount of energy associated with a food item by 85%, which is the typical amount of energy actually obtained by a human after respiration has been completed.[citation needed] In animal nutrition, where energy is a critical element of the economics of meat production, researchers may determine a specific metabolizable energy for each component (protein, fat, etc.) of each ingredient of the feed.
Nutrition labels[edit]
Many governments require food manufacturers to label the energy content of their products, to help consumers control their energy intake.[6] In the European Union, manufacturers of packaged food must label the nutritional energy of their products in both kilocalories and kilojoules, when required. In the United States, the equivalent mandatory labels display only "Calories",[7] often as a substitute for the name of the quantity being measured, food energy; an additional kilojoules figure is optional and is rarely used. In Australia and New Zealand, the food energy must be stated in kilojoules (and optionally in kilocalories as well), and other nutritional energy information is similarly conveyed in kilojoules.[8][9] The energy available from the respiration of food is usually given on labels for 100 g, for a typical serving size (according to the manufacturer), and/or for the entire pack contents.[citation needed]
The amount of food energy associated with a particular food could be measured by completely burning the dried food in a bomb calorimeter, a method known as direct calorimetry.[10] However, the values given on food labels are not determined in this way. The reason for this is that direct calorimetry also burns the dietary fiber, and so does not allow for fecal losses; thus direct calorimetry would give systematic overestimates of the amount of fuel that actually enters the blood through digestion. What are used instead are standardized chemical tests or an analysis of the recipe using reference tables for common ingredients[11] to estimate the product's digestible constituents (protein, carbohydrate, fat, etc.). These results are then converted into an equivalent energy value based on the following standardized table of energy densities.[4][12] However "energy density" is a misleading term for it once again assumes that energy is IN the particular food, whereas it simply means that "high density" food needs more oxygen during respiration, leading to greater transfer of energy.[1][13]
Note that the following standardized table of energy densities[12] is an approximation and the value in kJ/g does not convert exactly to kcal/g using a conversion factor.
| Food component | Energy density | |
|---|---|---|
| kJ/g | kcal/g | |
| Fat | 37 | 9 |
| Ethanol (drinking alcohol) | 29 | 7 |
| Proteins | 17 | 4 |
| Carbohydrates | 17 | 4 |
| Organic acids | 13 | 3 |
| Polyols (sugar alcohols, sweeteners) | 10 | 2.4 |
| Fiber | 8 | 2 |
All the other nutrients in food are noncaloric and are thus not counted.
Recommended daily intake[edit]
Increased mental activity has been linked with moderately increased brain energy consumption.[14] Older people and those with sedentary lifestylesrequire less energy; children and physically active people require more.
Recommendations in the United States are 10,900 and 8,400 kJ (2,600 and 2,000 kcal) for men and women (respectively) between 31 and 35, at a physical activity level equivalent to walking about 2.5 to 5 km (11⁄2 to 3 mi) per day at 5 to 6 km/h (3 to 4 mph) in addition to the light physical activity associated with typical day-to-day life,[15] with French guidance suggesting roughly the same levels.[16]
Recognizing that people of different age and gender groups have varying daily activity levels, Australia's National Health and Medical Research Councilrecommends no single daily energy intake, but instead prescribes an appropriate recommendation for each age and gender group.[17] Notwithstanding, nutrition labels on Australian food products typically recommend the average daily energy intake of 8,800 kJ (2,100 kcal).
According to the Food and Agriculture Organization of the United Nations, the average minimum energy requirement per person per day is about 7,500 kJ (1,800 kcal).[18]
List of countries by food energy intake[edit]
Energy usage in the human body[edit]
The human body uses the energy released by respiration for a wide range of purposes: about 20% of the energy is used for brain metabolism, and much of the rest is used for the basal metabolic requirements of other organs and tissues. In cold environments, metabolism may increase simply to produce heat to maintain body temperature. Among the diverse uses for energy, one is the production of mechanical energy by skeletal muscle to maintain posture and produce motion.
The conversion efficiency of energy from respiration into mechanical (physical) power depends on the type of food and on the type of physical energy usage (e.g., which muscles are used, whether the muscle is used aerobically or anaerobically). In general, the efficiency of muscles is rather low: only 18 to 26% of the energy available from respiration is converted into mechanical energy.[19] This low efficiency is the result of about 40% efficiency of generating ATP from the respiration of food, losses in converting energy from ATP into mechanical work inside the muscle, and mechanical losses inside the body. The latter two losses are dependent on the type of exercise and the type of muscle fibers being used (fast-twitch or slow-twitch). However, alterations in the structure of the material consumed can cause modifications in the amount of energy that can be derived from the food; i.e. caloric value depends on the surface area and volume of a food. For an overall efficiency of 20%, one watt of mechanical power is equivalent to 18 kJ/h (4.3 kcal/h). For example, a manufacturer of rowing equipment shows calories released from "burning" food as four times the actual mechanical work, plus 1,300 kJ (300 kcal) per hour,[20] which amounts to about 20% efficiency at 250 watts of mechanical output. It can take up to 20 hours of little physical output (e.g., walking) to "burn off" 17,000 kJ (4,000 kcal)[21] more than a body would otherwise consume. For reference, each kilogram of body fat is roughly equivalent to 32,300 kilojoules of food energy (i.e., 3,500 kilocalories per pound).[22]
In addition, the quality of calories matters because the energy absorption rate of different foods with equal amounts of calories may vary.[citation needed]Some nutrients have regulatory roles affected by cell signaling, in addition to providing energy for the body.[23] For example, leucine plays an important role in the regulation of protein metabolism and suppresses an individual's appetite.[24]
Swings in body temperature – either hotter or cooler – increase the metabolic rate, thus burning more energy. Prolonged exposure to extremely warm or very cold environments increases the basal metabolic rate (BMR). People who live in these types of settings often have BMRs 5–20% higher than those in other climates.[citation needed] Physical activity also significantly increases body temperature, which in turn uses more energy from respiration.[citation needed]
See also[edit]
- Atwater system
- Basal metabolic rate
- Calorie
- Chemical energy
- Food chain
- Food composition
- Heat of combustion
- Nutrition facts label
- Table of food nutrients
Calorimetry is the science or act of measuring changes in state variables of a body for the purpose of deriving the heat transfer associated with changes of its state due, for example, to chemical reactions, physical changes, or phase transitions under specified constraints. Calorimetry is performed with a calorimeter. The word calorimetry is derived from the Latin word calor, meaning heat and the Greek word μέτρον (metron), meaning measure. Scottish physician and scientist Joseph Black, who was the first to recognize the distinction between heat and temperature, is said to be the founder of the science of calorimetry.[2]
Indirect calorimetry calculates heat that living organisms produce by measuring either their production of carbon dioxide and nitrogen waste (frequently ammonia in aquatic organisms, or urea in terrestrial ones), or from their consumption of oxygen. Lavoisier noted in 1780 that heat production can be predicted from oxygen consumption this way, using multiple regression. The dynamic energy budget theory explains why this procedure is correct. Heat generated by living organisms may also be measured by direct calorimetry, in which the entire organism is placed inside the calorimeter for the measurement.
A widely used modern instrument is the differential scanning calorimeter, a device which allows thermal data to be obtained on small amounts of material. It involves heating the sample at a controlled rate and recording the heat flow either into or from the specimen.
https://en.wikipedia.org/wiki/Calorimetry
The standard atmosphere (symbol: atm) is a unit of pressure defined as 101325 Pa (1.01325 bar). It is sometimes used as a reference pressure or standard pressure. It is approximately equal to Earth's atmospheric pressure at sea level.
https://en.wikipedia.org/wiki/Standard_atmosphere_(unit)
In chemistry, bond energy (BE), also called the mean bond enthalpy[1] or average bond enthalpy[2] is the measure of bond strength in a chemical bond.[3] IUPAC defines bond energy as the average value of the gas-phase bond-dissociation energy (usually at a temperature of 298.15 K) for all bonds of the same type within the same chemical species.[4] The larger the average bond energy, per electron-pair bond, of a molecule, the more stable and lower-energy the molecule.[5]
The bond dissociation energy (enthalpy)[6] is also referred to as bond disruption energy, bond energy, bond strength, or binding energy (abbreviation: BDE, BE, or D). It is defined as the standard enthalpy change of the following fission: R - X → R + X. The BDE, denoted by Dº(R - X), is usually derived by the thermochemical equation,
The enthalpy of formation ΔHfº of a large number of atoms, free radicals, ions, clusters and compounds is available from the websites of NIST, NASA, CODATA, and IUPAC. Most authors prefer to use the BDE values at 298.15 K.
For example, the carbon–hydrogen bond energy in methane BE(C–H) is the enthalpy change (∆H) of breaking one molecule of methane into a carbon atom and four hydrogen radicals, divided by four. The exact value for a certain pair of bonded elements varies somewhat depending on the specific molecule, so tabulated bond energies are generally averages from a number of selected typical chemical species containing that type of bond.[7]
Bond energy (BE) is the average of all bond-dissociation energies of a single type of bond in a given molecule.[8] The bond-dissociation energies of several different bonds of the same type can vary even within a single molecule. For example, a water molecule is composed of two O–H bonds bonded as H–O–H. The bond energy for H2O is the average of energy required to break each of the two O–H bonds in sequence:
Although the two bonds are the equivalent in the original symmetric molecule, the bond-dissociation energy of an oxygen–hydrogen bond varies slightly depending on whether or not there is another hydrogen atom bonded to the oxygen atom.
When the bond is broken, the bonding electron pair will split equally to the products. This process is called homolytic bond cleavage (homolytic cleavage; homolysis) and results in the formation of radicals.[9]
Predicting the bond strength by radius[edit]
Metallic radius, ionic radius, and covalent radius of each atom in a molecule can be used to estimate the bond strength. For example, the covalentradius of boron is estimated at 83.0 pm, but the bond length of B–B in B2Cl4 is 175 pm, a significantly larger value. This would indicate that the bond between the two boron atoms is a rather weak single bond. In another example, the metallic radius of rhenium is 137.5 pm, with a Re–Re bond length of 224 pm in the compound Re2Cl8. From this data, we can conclude that the bond is a very strong bond or a quadruple bond. This method of determination is most useful for covalently bonded compounds.[10]
Factors affecting ionic bond energy[edit]
The electronegativity of the two atoms bonding together affects ionic bond energy.[11] The farther away the electronegativity of 2 atoms, the stronger the bond generally. For example, Cesium has the lowest, and Fluorine has the highest and the make the strongest ionic bond (well single bond at least). Assuming the strongest polar covalent is the Carbon-Fluorine bond. And mostly, ionic bonds are stronger than covalent bonds. By checking at melting points, ionic compounds have high melting points and covalent compounds have low melting points.[12]
See also[edit]
Enthalpy /ˈɛnθəlpi/ (![]() listen) is a property of a thermodynamic system, and is defined as the sum of the system's internal energy and the product of its pressure and volume.[1][2] It is a state function used in many measurements in chemical, biological, and physical systems at a constant pressure, which is conveniently provided by the large ambient atmosphere. The pressure–volume term expresses the workrequired to establish the system's physical dimensions, i.e. to make room for it by displacing its surroundings.[3][4] The pressure-volume term is very small for solids and liquids at common conditions, and fairly small for gases. Therefore, enthalpy is a stand-in for energy in chemical systems; bond, lattice, solvation and other "energies" in chemistry are actually enthalpy differences. As a state function, enthalpy depends only on the final configuration of internal energy, pressure, and volume, not on the path taken to achieve it.
listen) is a property of a thermodynamic system, and is defined as the sum of the system's internal energy and the product of its pressure and volume.[1][2] It is a state function used in many measurements in chemical, biological, and physical systems at a constant pressure, which is conveniently provided by the large ambient atmosphere. The pressure–volume term expresses the workrequired to establish the system's physical dimensions, i.e. to make room for it by displacing its surroundings.[3][4] The pressure-volume term is very small for solids and liquids at common conditions, and fairly small for gases. Therefore, enthalpy is a stand-in for energy in chemical systems; bond, lattice, solvation and other "energies" in chemistry are actually enthalpy differences. As a state function, enthalpy depends only on the final configuration of internal energy, pressure, and volume, not on the path taken to achieve it.
The unit of measurement for enthalpy in the International System of Units (SI) is the joule. Other historical conventional units still in use include the calorie and the British thermal unit (BTU).
The total enthalpy of a system cannot be measured directly because the internal energy contains components that are unknown, not easily accessible, or are not of interest in thermodynamics. In practice, a change in enthalpy is the preferred expression for measurements at constant pressure, because it simplifies the description of energy transfer. When transfer of matter into or out of the system is also prevented and no electrical or shaft work is done, at constant pressure the enthalpy change equals the energy exchanged with the environment by heat.
In chemistry, the standard enthalpy of reaction is the enthalpy change when reactants in their standard states (p = 1 bar; usually T = 298 K) change to products in their standard states.[5] This quantity is the standard heat of reaction at constant pressure and temperature, but it can be measured by calorimetricmethods even if the temperature does vary during the measurement, provided that the initial and final pressure and temperature correspond to the standard state. The value does not depend on the path from initial to final state since enthalpy is a state function.
Enthalpies of chemical substances are usually listed for 1 bar (100 kPa) pressure as a standard state. The temperature does not have to be specified, but tables generally list the standard heat of formation at 25 °C (298 K). For endothermic (heat-absorbing) processes, the change ΔH is a positive value; for exothermic(heat-releasing) processes it is negative.
The enthalpy of an ideal gas is independent of its pressure or volume, and depends only on its temperature, which correlates to its thermal energy. Real gases at common temperatures and pressures often closely approximate this behavior, which simplifies practical thermodynamic design and analysis.
https://en.wikipedia.org/wiki/Enthalpy
The joule (/dʒaʊl, dʒuːl/ jowl, jool;[1][2][3] symbol: J) is a derived unit of energy in the International System of Units.[4] It is equal to the energy transferred to (or work done on) an object when a force of one newtonacts on that object in the direction of the force's motion through a distance of one metre (1 newton-metre or N⋅m). It is also the energy dissipated as heat when an electric current of one ampere passes through a resistance of one ohm for one second. It is named after the English physicist James Prescott Joule(1818–1889).[5][6][7]
Watt-second[edit]
A watt-second (symbol W s or W·s) is a derived unit of energy equivalent to the joule.[25] The watt-second is the energy equivalent to the power of one watt sustained for one second. While the watt-second is equivalent to the joule in both units and meaning, there are some contexts in which the term "watt-second" is used instead of "joule".[why?]
Photography[edit]
In photography, the unit for flashes is the watt-second. A flash can be rated in watt-seconds (e.g., 300 W⋅s) or in joules (different names for the same thing), but historically, the term "watt-second" has been used and continues to be used.
The energy rating a flash is given is not a reliable benchmark for its light output because there are numerous factors that affect the energy conversion efficiency. For example, the construction of the tube will affect the efficiency, and the use of reflectors and filters will change the usable light output towards the subject. Some companies specify their products in "true" watt-seconds, and some specify their products in "nominal" watt-seconds.[26]
See also[edit]
https://en.wikipedia.org/wiki/Joule
In physics, energy is the quantitative property that must be transferred to a body or physical system to perform work on the body, or to heat it. Energy is a conserved quantity; the law of conservation of energystates that energy can be converted in form, but not created or destroyed. The unit of measurement in the International System of Units (SI) of energy is the joule, which is the energy transferred to an object by the work of moving it a distance of one metre against a force of one newton.
Common forms of energy include the kinetic energy of a moving object, the potential energy stored by an object's position in a force field (gravitational, electric or magnetic), the elastic energy stored by stretching solid objects, the chemical energy released when a fuel burns, the radiant energy carried by light, and the thermal energy due to an object's temperature.
Mass and energy are closely related. Due to mass–energy equivalence, any object that has mass when stationary (called rest mass) also has an equivalent amount of energy whose form is called rest energy, and any additional energy (of any form) acquired by the object above that rest energy will increase the object's total mass just as it increases its total energy. For example, after heating an object, its increase in energy could be measured as a small increase in mass, with a sensitive enough scale.
Living organisms require energy to stay alive, such as the energy humans get from food. Human civilization requires energy to function, which it gets from energy resources such as fossil fuels, nuclear fuel, or renewable energy. The processes of Earth's climate and ecosystem are driven by the radiant energy Earth receives from the Sun and the geothermal energy contained within the earth.
https://en.wikipedia.org/wiki/Energy
The following outline is provided as an overview of and topical guide to energy:
Energy – in physics, this is an indirectly observed quantity often understood as the ability of a physical system to do work on other physical systems.[1][2] Since work is defined as a force acting through a distance (a length of space), energy is always equivalent to the ability to exert force (a pull or a push) against an object that is moving along a definite path of certain length.
Forms of energy[edit]
- Chemical energy – energy contained in molecules
- Electrical energy – energy from electric fields
- Electro-centric energy – energy sustaining the continuous motion of free electrons.[1]
- Gravitational energy – energy from gravitational fields
- Ionization energy – energy that binds an electron to its atom or molecule
- Kinetic energy – (≥0), energy of the motion of a body
- Magnetic energy – energy from magnetic fields
- Mechanical energy – The sum of (usually macroscopic) kinetic and potential energies
- Mechanical wave – (≥0), a form of mechanical energy propagated by a material's oscillations
- Nuclear binding energy – energy that binds nucleons to form the atomic nucleus
- Potential energy – energy possessed by a body by virtue of its position relative to others, stresses within itself, electric charge, and other factors.[3][4]
- Elastic energy – energy of deformation of a material (or its container) exhibiting a restorative force
- Gravitational energy – potential energy associated with a gravitational field.
- Nuclear potential energy
- Radiant energy – (≥0), energy of electromagnetic radiation including light
- Rest energy – (≥0) that E=mc² an object's rest mass
- Surface energy
- Thermal energy – a microscopic, disordered equivalent of mechanical energy
- Heat – an amount of thermal energy being transferred (in a given process) in the direction of decreasing temperature
- Work (physics) – an amount of energy being energy transfer in a given Process (thermodynamic) due to displacement in the direction of an applied force
Measurement[edit]
Units[edit]
List of common units for energy. Official or common symbol in brackets after name and exact or approximate value of unit in joule in brackets after description.
SI unit[edit]
Other metric units[edit]
- Kilowatt-hour (kW·h) – corresponds to one kilowatt of power being used over a period of one hour (3.6 MJ).
- Calorie (cal) – equal to the energy need to raise the temperature of one gram of water by one degree Celsius (~4.184 J).
- Erg (erg) – unit of energy and mechanical work in the centimetre-gram-second (CGS) system of units (10−7 J).
Imperial or US Customary units[edit]
- British thermal unit (BTU) – equal to the energy need to raise the temperature of one pound of water by one degree Fahrenheit (~1055 J).
- Therm (thm) – unit of heat energy. In the US gas industry it is defined as exactly 100,000 BTU59 °F. It is approximately the heat equivalent of burning 100 cubic feet (2.8 m3) of natural gas (~105.5 MJ).
- Quad – unit of energy equal to 1015 (a short-scale quadrillion) BTU.
- Foot-pound (ft·lbf or ft·lbf) – unit of mechanical work, or energy, although in scientific fields one commonly uses joule (~1.356 J).
Other units[edit]
- Electronvolt (eV) – the amount of energy gained by a single unbound electron when it falls through an electrostatic potential difference of one volt (~1.60 × 10−19 J).
- Planck energy (EP) – natural unit of energy common in particle physics (~1.96×109 J).
- Barrel of oil equivalent (BOE) – energy unit equal to the energy released when burning one barrel (159 litres) of oil (~6.12 GJ).
- Tonne of oil equivalent (toe) – energy unit equal to the energy released when burning one tonne of oil (~42 GJ).
Related units and concepts[edit]
- Volt
- Ampere
- Coulomb
- Enthalpy
- EU energy label
- Fill factor – defined as the ratio of the maximum power (Vmp x Jmp) divided by the short-circuit current (Isc) and open-circuit voltage (Voc) in light current density – voltage (J-V) characteristics of solar cells.
- Gigaton – Metric Unit of mass, equal to 1,000,000,000 (1 billion) metric tons, 1,000,000,000,000 (1 trillion) kilograms
- Any of various units of energy, such as gigatons of TNT equivalent, gigatons of coal equivalent, gigatons petroleum equivalent.
- Gray (unit) – (symbol: Gy), is the SI unit of energy for the absorbed dose of radiation. One gray is the absorption of one joule of radiation energy by one kilogram of matter. One gray equals 100 rad, an older unit.
- Heat
- Mass-energy equivalence – where mass has an energy equivalence, and energy has a mass equivalence
- Megawatt
- Net energy gain
- Power factor – of an AC electric power system is defined as the ratio of the real power to the apparent power.
Energy industry[edit]
- Worldwide energy supply, outline by country/region
- World energy resources and consumption
- List of energy resources, substances like fuels, petroleum products and electricity
- Energy crisis, the need to conserve energy resources
- Energy development, development of energy resources — ongoing effort to provide abundant and accessible energy, through knowledge, skills and construction
- Embodied energy, the sum total of energy expended to deliver a good or service as it travels through the economy
- Energy conservation, tips for conserving energy resources
- Energy economics, as the foundation of other relationships
- Energy policy, government policies and plans for energy supply
- Energy storage, methods commonly used to store energy resources for later use
- Energy system, an interpretation the energy sector in system terms
- Biosphere
- Ecological energetics
- Ecology
- Energy balance
- Earth Day
- Energy speculation
- Free energy suppression
- Future energy development – Provides a general overview of future energy development.
- History of perpetual motion machines
- Hubbert peak theory, also known as peak oil – the theory that world oil production will peak (or has peaked), and will then rapidly decline, with a corresponding rapid increase in prices.
- Primary production
- Power harvesting
- Renewable energy development
Energy infrastructure[edit]
See especially Category:Electric power and Category:Fuels for a large number of conventional energy related topics.
- Energy storage
- Electricity generation
- Electricity retailing
- Grid energy storage
- Liquified natural gas
- Microwave power transmission
- Power station
- Power supply
- Power transmission
- Underground power station
Energy applications[edit]
- Biofuel
- Distributed generation
- Electric vehicle
- Hybrid vehicle
- Hydrogen vehicle
- Passive solar building design
- Steam engine
History of energy[edit]
- History of the energy industry
Physics of energy[edit]
- Energy
- Activation energy, explains the differences in the speeds of various chemical reactions
- Bioenergetics
- Chemical energetics
- Energy in physical cosmology
- Energy in Earth science that is responsible for the macroscopic transformations on the planet Earth
- Electricity
- Exergy
- Green energy
- Orders of magnitude (energy), list describing various energy levels between 10−31 joules and 1070 joules
- Thermodynamics
- Perpetual motion
- Heat
- History of energy
- Forms of energy, the forms in which energy can be defined
- Energy transformation, relating to energy's changes from one form to another.
- Energy (signal processing), the inner product of a signal in the time domain
- Energy density spectrum, relating to the distribution of signal energy over frequencies.
- Potential energy, the form of energy that is due to position of an object
- Kinetic energy, the form of energy as a consequence of the motion of an object or its constituents
- Mechanical energy, the potential energy and kinetic energy present in the components of a mechanical system
- Binding energy, a concept explaining how the constituents of atoms or molecules are bound together
- Bond energy, a measure of the strength of a chemical bond
- Nuclear energy, energy that is the consequence of decomposition or combination of atomic nuclei
- Osmotic power, also salinity gradient power or blue energy, the energy available from the difference in the salt concentration between seawater and river water
- Gibbs free energy, a related concept in chemical thermodynamics that incorporates entropy considerations
- Helmholtz free energy, a thermodynamic potential that measures the "useful" work obtainable from a closed thermodynamic system at a constant temperature, useful for studying explosive chemical reactions
- Elastic energy, which causes or is released by the elastic distortion of a solid or a fluid
- Ionization energy (IE), the energy required to strip an atom of an electron
- Interaction energy, the contribution to the total energy that is a result of interaction between the objects being considered
- Internal energy (abbreviated E or U), the total kinetic energy due to the motion of molecules (translational, rotational, vibrational) and the total potential energy associated with the vibrational and electric energy of atoms within molecules.
- Negative energy
- Energy conversion, process of converting energy from one form to another
- Dark energy, used to explain some cosmological phenomena
- Energy quality, empirical experience of the characteristics of different energy forms as they flow and transform
- Energy density, amount of energy stored in a given system or region of space per unit volume, or per unit mass
- Energy flow, flow of energy in an ecosystem through food chains
- Energetics (disambiguation), the scientific study of energy in general
- Stress–energy tensor, the density and flux of energy and momentum in space-time; the source of the gravitational field in general relativity
- Food energy, energy in food that is available
- Primary energy, energy contained in raw fuels and any other forms of energy received by a system as input to the system.
- Radiant energy, energy that is transported by waves
- Rotational energy, part of an object's total kinetic energy due to its rotation
- Solar radiation, radiant energy emitted by the sun, particularly electromagnetic energy
- Tidal power, also called tidal energy, is a form of hydropower that converts the energy of tides into useful forms of power - mainly electricity, dynamic tidal power, tidal lagoons, tidal barrages
- Wave power is the transport of energy by ocean surface waves, and the capture of that energy to do useful work — for example, electricity generation, water desalination, or the pumping of water (into reservoirs). Machinery able to exploit wave power is generally known as a wave energy converter (WEC).
- Wind energy is the kinetic energy of air in motion;Wind power is the conversion of wind energy into a useful form of energy, such as using wind turbines to make electricity, windmills for mechanical power, windpumps for water pumping or drainage, or sails to propel ships
Allegorical and esoteric[edit]
- Energy (esotericism), invoked by spiritualists for alternative modes of healing the human body as well as a spirit that permeates all of reality.
- Orgone, Wilhelm Reich discovered this energy and tried to use it to cure various physical ailments and control the weather.
- Bioenergetic analysis, body-oriented Reichian psychotherapy
- Qi, a concept from Oriental medicine that is sometimes translated as "energy" in the West.
- Vitalism, often referred to as "energy"
- Cold fusion, nuclear fusion at conditions close to room temperature.
- Bubble fusion, also known as Sonofusion, energy from acoustic collapse of bubbles.
- Water-fuelled car, powering a car using water as fuel.
Politics[edit]
Energy issues[edit]
- 2000 Watt society
- Environmental concerns with electricity generation
- Fuel poverty
- Greasestock, American showcase of vehicles and technologies powered by alternative energy
- Low-carbon economy
- Decarbonisation plans that get to zero CO2 emissions
- Peak Oil
- Soft energy path – an energy use and development strategy delineated and promoted by some energy experts and activists
- Strategic Petroleum Reserve (disambiguation)
Energy policies and use – national and international[edit]
International[edit]
- Energy policy – an introductory article
- Energy and Environmental Security Initiative
Regional and national[edit]
- Energy law – overview of many energy laws from various countries and states
- Energy Tax Act – United States energy-related legislation. See also : Category:United States federal energy legislation
- United Kingdom:
Economics[edit]
Energy companies[edit]
- Exxon Mobil
- Enercon GmbH – Company based in Germany that operates in the wind turbine industry. One of the biggest producers in the world.
- Saudi Aramco
- Sasol
- United States Enrichment Corporation – contracts with the United States Department of Energy to produce enriched uranium.
Non-profit organizations[edit]
Industry associations[edit]
- OPEC – Organization of Petroleum-exporting Countries
- IEA – International Energy Agency
- CAPP – Canadian Association of Petroleum Producers
- World LP Gas Association – WLPGA
Innovators[edit]
- Alessandro Volta
- Charles Kettering
- Farrington Daniels – solar energy
- Georges Leclanché – battery
- John Frederic Daniell – Daniell cell
- Rudolf Diesel – compression ignition internal combustion engine
- Georges Imbert – wood gas
- Leonardo da Vinci
- Moritz von Jacobi
- Nicolaus Otto – internal combustion engine
- Robert Stirling – Stirling engine (external combustion)
- Nikola Tesla
- James Watt – steam engine with separate condensor
Lists[edit]
- List of books about energy issues
- List of energy abbreviations
- List of energy storage projects
- List of large wind farms
- List of notable renewable energy organizations
- List of photovoltaics companies
- List of renewable energy topics by country
- List of solar thermal power stations
- Index of wave articles
- List of wind turbine manufacturers
See also[edit]
https://en.wikipedia.org/wiki/Outline_of_energy
Calorie, calorie, kcal, Joule, etc..
Heat, Enthalpy, Energy, Entropy
Measure, Measurement, Evidence
Observations
The nutrition facts label (also known as the nutrition information panel, and other slight variations) is a label required on most packaged food in many countries, showing what nutrients and other ingredients (to limit and get enough of) are in the food. Labels are usually based on official nutritional rating systems. Most countries also release overall nutrition guides for general educational purposes. In some cases, the guides are based on different dietary targets for various nutrients than the labels on specific foods.
Nutrition facts labels are one of many types of food labels required by regulation or applied by manufacturers.
United States[edit]
Description[edit]
In the United States, the Nutritional Facts label lists the percentage supplied that is recommended to be met, or to be limited, in one day of human nutrients based on a daily diet of 2,000 calories.
With certain exceptions, such as babies foods, the following Daily Values are used.[21] These are called Reference Daily Intake (RDI) values and were originally based on the highest 1968 Recommended Dietary Allowances (RDA) for each nutrient in order to assure that the needs of all age and sex combinations were met.[22] These are older than the current Recommended Dietary Allowances of the Dietary Reference Intake. For vitamin C, vitamin D, vitamin E, vitamin K, calcium, phosphorus, magnesium, and manganese, the current highest RDAs are up to 50% higher than the older Daily Values used in labeling, whereas for other nutrients the recommended needs have gone down. A side-by-side table of the old and new adult Daily Values is provided at Reference Daily Intake. As of October 2010, the only micronutrients that are required to be included on all labels are vitamin A, vitamin C, calcium, and iron.[23] To determine the nutrient levels in the foods, companies may develop or use databases, and these may be submitted voluntarily to the U.S. Food and Drug Administration for review.[24]
| Nutrient | Daily Value for label (before 2016 update) | highest RDA of DRI | unit |
|---|---|---|---|
| Vitamin A | 5,000 | 3,000 | IU |
| Vitamin C | 60 | 90 | mg |
| Thiamin | 1.5 | 1.2 | mg |
| Riboflavin | 1.7 | 1.3 | mg |
| Niacin | 20 | 16 | mg |
| Pantothenic acid | 10 | 5 | mg |
| Vitamin B6 | 2 | 1.7 | mg |
| Folate | 400 | 400 | μg |
| Biotin | 300 | 30 | μg |
| Vitamin B12 | 6 | 2.4 | μg |
| Vitamin D | 400 | 600 | IU |
| Vitamin E | 12 | 15 | mg |
| Vitamin K | 80 | 120 | μg |
| Calcium | 1,000 | 1,300 | mg |
| Iron | 18 | 18 | mg |
| Phosphorus | 1,000 | 1,250 | mg |
| Iodine | 150 | 150 | μg |
| Magnesium | 400 | 420 | mg |
| Zinc | 15 | 11 | mg |
| Selenium | 70 | 55 | μg |
| Copper | 2 | 0.9 | mg |
| Manganese | 2 | 2.3 | mg |
| Chromium | 120 | 35 | μg |
| Molybdenum | 75 | 45 | μg |
| Chloride | 3,400 | 2,300 | mg |
Additionally, there is a requirement for ingredients to be listed in order from highest to lowest quantity, according to their weight.[25] This requirement has some flexibility during the COVID-19 pandemic.[26][27]
The label was mandated for most food products under the provisions of the 1990 Nutrition Labeling and Education Act (NLEA), per the recommendations of the U.S. Food and Drug Administration.[28] It was one of several controversial actions taken during the tenure of FDA Commissioner Dr. David Kessler. The law required food companies to begin using the new food label on packaged foods beginning May 8, 1994. (Meat and poultry products were not covered by NLEA, though the U.S. Department of Agriculture proposed similar regulations for voluntary labeling of raw meat and poultry.[29]) Foods labeled before that day could use the old label. This appeared on all products in 1995. The old label was titled "Nutrition Information Per Serving" or simply, "Nutrition Information".
The label begins with a standard serving measurement, calories are listed second, and then following is a breakdown of the constituent elements including % daily value (%DV).[30] Always listed are total fat, sodium, carbohydrates and protein; the other nutrients usually shown may be suppressed, if they are zero. Usually all 15 nutrients are shown: calories, calories from fat, fat, saturated fat, trans fat, cholesterol, sodium, carbohydrates, dietary fiber, sugars, protein, vitamin A, vitamin C, calcium, and iron.
Products containing less than 5 g of fat show amounts rounded to the nearest 0.5 g. Amounts less than 0.5 g are rounded to 0 g. For example, if a product contains 0.45 g of trans fat per serving, and the package contains 18 servings, the label would show 0 g of trans fat, even though the product actually contains a total of 8.1 g of trans fat.
In addition to the nutrition label, products may display certain nutrition information or health claims on packaging. These health claims are only allowed by the FDA for "eight diet and health relationships based on proven scientific evidence", including: calcium and osteoporosis, fiber-containing grain products, fruits and vegetables and cancer, fruits, vegetables, and grain products that contain fiber—particularly soluble fiber—and the risk of coronary heart disease, fat and cancer, saturated fat and cholesterol and coronary heart disease, sodium and hypertension, and folate and neural tube defects.[31] The Institute of Medicine recommended these labels contain the most useful nutritional information for consumers: saturated fats, trans fats, sodium, calories, and serving size.[32] In January 2011, food manufacturers and grocery stores announced plans to display some of this nutrition information on processed food.[33]
The nutrition facts label currently appears on more than 6.5 billion food packages. President Bill Clinton issued an award of design excellence for the nutrition facts label in 1997 to Burkey Belser in Washington, DC.[34]
The FDA does not require any specific typeface be used in the Nutrition Facts label, mandating only that the label "utilize a single easy-to-read type style",[35] though its example label uses Helvetica.[36] However, as regulated by the FDA and the USDA, it is mandatory for certain information listed in the label to be written in English, including: name of the product, net quantity, serving size and number of servings per package, nutrition facts, ingredient list, and name of manufacturer or distributor.[37] The smallest lettering should be at least 1/16th of an inch tall (1.5875 millimeters), based on the height of a lowercase "o".[38]
In January 2006, Trans fat was required to be listed under saturated fat. This was the first significant change to the Nutrition Facts panel since it was introduced in 1993.[39]
2016 revision[edit]
In 2014, the U.S. Food and Drug Administration proposed several simultaneous improvements to nutrition labeling for the first time in over 20 years.[40][41] The proposed changes were based on trends of consumption of nutrients of public health importance.[42] However, studies had shown that the majority of the U.S. population could not understand the information in the then current Nutrition Facts Label.[43] Nutrition label numeracy is particularly low in older individuals, of black and Hispanic race/ethnicity, who are unemployed, born outside of the US, have lower English proficiency, lower education achievement, lower income, or live in the South.[44]
Final changes included raising serving sizes to more accurately reflect how many servings the average individual is actually consuming, removing "calories from fat" and instead focusing on total calories and type of fats being consumed in a product, and listing extra sugar added to a product, as well as declaring the amount of Vitamin D and potassium in a product and adjusting recommended Daily Value amounts.[40][45][42] Some of these changes sparked a major debate between the food industry and public health agencies. The proposal to indicate sugar added during food production, in particular, was brought forward by the FDA as a measure to counter the increase in per capita sugar consumption in the US, which over the last decades exceeded the limits recommended by scientific institutions and governmental agencies.[46][47] Major American food associations opposed the label change, indicating "lack of merit" and "no preponderance of evidence" to justify the inclusion of sugar added in the new label.[48][49]
The rules for the new design were finalized on May 20, 2016. Manufacturers were initially given until July 26, 2018 to comply (or July 26, 2019 if they have less than $10 million in annual food sales);[50] a rule change extend the compliance deadline to January 1, 2020 (or January 1, 2021 for smaller sellers).[51][42] For food and dietary supplement labeling purposes the amounts of vitamins and nutritionally essential minerals in a serving are expressed as a percent of Daily Value (%DV). Many of the definitions of 100% Daily Value were changed as part of the revision.[52] A table of the old and new adult Daily Values is provided at Reference Daily Intake.
See also[edit]
- Diet (nutrition)
- Food energy
- List of food labeling regulations
- Table of food nutrients
- Nutrition scale
- Serving size
- Atwater system (for calculating available food energy)
- The Non-GMO Project
- Quack Miranda warning
https://en.wikipedia.org/wiki/Nutrition_facts_label
The Atwater system,[1] named after Wilbur Olin Atwater, or derivatives of this system are used for the calculation of the available energy of foods. The system was developed largely from the experimental studies of Atwater and his colleagues in the later part of the 19th century and the early years of the 20th at Wesleyan University in Middletown, Connecticut. Its use has frequently been the cause of dispute, but few alternatives have been proposed. As with the calculation of protein from total nitrogen, the Atwater system is a convention and its limitations can be seen in its derivation.
Derivation[edit]
Available energy (as used by Atwater) is equivalent to the modern usage of the term metabolisable energy (ME).
In most studies on humans, losses in secretions and gases are ignored. The gross energy (GE) of a food, as measured by bomb calorimetry is equal to the sum of the heats of combustion of the components – protein (GEp), fat (GEf) and carbohydrate (GEcho) (by difference) in the proximate system.
Atwater considered the energy value of feces in the same way.
By measuring coefficients of availability or in modern terminology apparent digestibility, Atwater derived a system for calculating faecal energy losses.
where Dp, Df, and Dcho are respectively the digestibility coefficients of protein, fat and carbohydrate calculated as
for the constituent in question.
Urinary losses were calculated from the energy to nitrogen ratio in urine. Experimentally this was 7.9 kcal/g (33 kJ/g) urinary nitrogen and thus his equation for metabolisable energy became
Gross energy values[edit]
Atwater collected values from the literature and also measured the heat of combustion of proteins, fats and carbohydrates. These vary slightly depending on sources and Atwater derived weighted values for the gross heat of combustion of the protein, fat and carbohydrate in the typical mixed diet of his time. It has been argued that these weighted values are invalid for individual foods and for diets whose composition in terms of foodstuffs is different from those eaten in the US in the early 20th century.
Apparent digestibility coefficients[edit]
Atwater measured a large number of digestibility coefficients for simple mixtures, and in substitution experiments derived values for individual foods. These he combined in a weighted fashion to derive values for mixed diets. When these were tested experimentally with mixed diets they did not give a good prediction, and Atwater adjusted the coefficients for mixed diets.
Urinary correction[edit]
The energy/nitrogen ratio in urine shows considerable variation and the energy/organic matter is less variable, but the energy/nitrogen value provided Atwater with a workable approach although this has caused some confusion and only applies for subjects in nitrogen balance.
See also[edit]
https://en.wikipedia.org/wiki/Atwater_system#Gross_energy_values
https://en.wikipedia.org/wiki/Category:Food_science
https://en.wikipedia.org/wiki/Category:Nutrition
The United States Department of Agriculture (USDA), also known as the Agriculture Department, is the federal executive department responsible for developing and executing federal laws related to farming, forestry, rural economic development, and food. It aims to meet the needs of commercial farming and livestock food production, promotes agricultural trade and production, works to assure food safety, protects natural resources, fosters rural communities and works to end hunger in the United States and internationally.
Approximately 80% of the USDA's $141 billion budget goes to the Food and Nutrition Service (FNS) program. The largest component of the FNS budget is the Supplemental Nutrition Assistance Program(formerly known as the Food Stamp program), which is the cornerstone of USDA's nutrition assistance.[2]The United States Forest Service is the largest agency within the department, which administers national forests and national grasslands that together comprise about 25% of federal lands.
The Secretary of Agriculture is Tom Vilsack since February 24, 2021.
https://en.wikipedia.org/wiki/United_States_Department_of_Agriculture
A veterinarian (vet), also known as a veterinary surgeon or veterinary physician, is a medical professional who practices veterinary medicine by treating diseases, disorders, and injuries in non-human animals.
https://en.wikipedia.org/wiki/Veterinarian
Dentistry, also known as dental medicine and oral medicine, is a branch of medicine that consists of the study, diagnosis, prevention, and treatment of diseases, disorders, and conditions of the oral cavity(the mouth), commonly in the dentition (development and arrangement of teeth) as well as the oral mucosa, and of adjacent and related structures and tissues, particularly in associated maxillofacial (jaw and facial) area.[1] The field of dentistry or dental medicine includes teeth as well as other aspects of the craniofacial complex including the temporomandibular joint and other supporting, muscular, lymphatic, nervous, vascular, and anatomical structures. The practitioner is called a dentist.
https://en.wikipedia.org/wiki/Dentistry
Medicine is the science[1] and practice[2] of caring for a patient, managing the diagnosis, prognosis, prevention, treatment, palliation of their injury or disease, and promoting their health. Medicine encompasses a variety of health care practices evolved to maintain and restore health by the preventionand treatment of illness.
https://en.wikipedia.org/wiki/Medicine
https://en.wikipedia.org/wiki/infectious_disease
https://en.wikipedia.org/wiki/emergency_medicine
https://en.wikipedia.org/wiki/general_medicine
https://en.wikipedia.org/wiki/antibiotic
Pressure (symbol: p or P) is the force applied perpendicular to the surface of an object per unit area over which that force is distributed.: 445 [1] Gauge pressure (also spelled gage pressure)[a] is the pressure relative to the ambient pressure.
Various units are used to express pressure. Some of these derive from a unit of force divided by a unit of area; the SI unit of pressure, the pascal (Pa), for example, is one newton per square metre (N/m2); similarly, the pound-force per square inch (psi) is the traditional unit of pressure in the imperial and U.S. customary systems. Pressure may also be expressed in terms of standard atmospheric pressure; the atmosphere (atm) is equal to this pressure, and the torr is defined as 1⁄760 of this. Manometric units such as the centimetre of water, millimetre of mercury, and inch of mercury are used to express pressures in terms of the height of column of a particular fluid in a manometer.
https://en.wikipedia.org/wiki/Pressure
Junction, matrix, connexion, connecting cell, interspace, interstitial matrix, etc..
https://en.wikipedia.org/wiki/Extracellular_matrix
biofilm, plaque, tartar, calculus, mineralization liquefication ; cavitation mole bone hole bone
bone teeth ; eye hair ; limb structure




























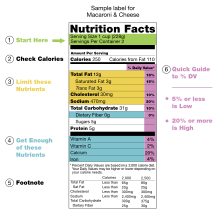








No comments:
Post a Comment