| Part of a series on |
| Water salinity |
|---|
 |
| Salinity levels |
|
Fresh water (< 0.05%) Brackish water (0.05–3%) Saline water (3–5%) Brine (> 5% up to 26%–28% max) |
| Bodies of water |
A hypersaline lake is a landlocked body of water that contains significant concentrations of sodium chloride, brines, and other salts, with saline levels surpassing that of ocean water (3.5%, i.e. 35 grams per litre or 0.29 pounds per US gallon).
Specific microbial species can thrive in high-salinity environments[1] that are inhospitable to most lifeforms,[2] including some that are thought to contribute to the colour of pink lakes.[3][4] Some of these species enter a dormant state when desiccated, and some species are thought to survive for over 250 million years.[2]
The water of hypersaline lakes has great buoyancy due to its high salt content.[5]
Hypersaline lakes are found on every continent, especially in arid or semi-arid regions.[1]
In the Arctic, the Canadian Devon Ice Cap contains two subglacial lakes that are hypersaline.[6] In Antarctica, there are larger hypersaline water bodies, lakes in the McMurdo Dry Valleys such as Lake Vanda with salinity of over 35% (i.e. 10 times as salty as ocean water).[citation needed]
The most saline water body in the world is the Gaet'ale Pond, located in the Danakil Depression in Afar, Ethiopia. The water of Gaet'ale Pond has a salinity of 43%, making it the saltiest water body on Earth[7] (i.e. 12 times as salty as ocean water). Previously, it was considered that the most saline lake outside of Antarctica was Lake Assal,[8] in Djibouti, which has a salinity of 34.8% (i.e. 10 times as salty as ocean water). Probably the best-known hypersaline lakes are the Dead Sea (34.2% salinity in 2010) and the Great Salt Lake in the state of Utah, US (5–27% variable salinity). The Dead Sea, dividing Israel and the West Bank from Jordan, is the world's deepest hypersaline lake. The Great Salt Lake, while having nearly three times the surface area of the Dead Sea, is shallower and experiences much greater fluctuations in salinity. At its lowest recorded water levels, it approaches 7.7 times the salinity of ocean water, but when its levels are high, its salinity drops to only slightly higher than that of the ocean.[9][10][11]
https://en.wikipedia.org/wiki/Hypersaline_lake
A subglacial lake is a lake that is found under a glacier, typically beneath an ice cap or ice sheet. Subglacial lakes form at the boundary between ice and the underlying bedrock, where gravitational pressure decreases the pressure melting point of ice.[1][2] Over time, the overlying ice gradually melts at a rate of a few millimeters per year.[3] Meltwater flows from regions of high to low hydraulic pressure under the ice and pools, creating a body of liquid water that can be isolated from the external environment for millions of years.[1][4]
Since the first discoveries of subglacial lakes under the Antarctic Ice Sheet, more than 400 subglacial lakes have been discovered in Antarctica, beneath the Greenland Ice Sheet, and under Iceland's Vatnajökull ice cap.[5][6][7] Subglacial lakes contain a substantial proportion of Earth's liquid freshwater, with the volume of Antarctic subglacial lakes alone estimated to be about 10,000 km3, or about 15% of all liquid freshwater on Earth.[8]
As ecosystems isolated from Earth's atmosphere, subglacial lakes are influenced by interactions between ice, water, sediments, and organisms. They contain active biological communities of extremophilic microbes that are adapted to cold, low-nutrient conditions and facilitate biogeochemical cycles independent of energy inputs from the sun.[9] Subglacial lakes and their inhabitants are of particular interest in the field of astrobiology and the search for extraterrestrial life.[10][11]
https://en.wikipedia.org/wiki/Subglacial_lake
The pressure melting point of ice is the temperature at which ice melts at a given pressure. The pressure melting point is nearly a constant 0 °C at pressures above the triple point at 611.7 Pa, where water can exist in only the solid or liquid phases, through atmospheric pressure (100 kPa) until about 10 MPa. With increasing pressure above 10 MPa, the pressure melting point decreases to a minimum of −21.9 °C at 209.9 MPa. Thereafter, the pressure melting point rises rapidly with pressure, passing back through 0 °C at 632.4 MPa.[1]
Pressure melting point in glaciers
Glaciers are subject to geothermal heat flux from below and atmospheric warming or cooling from above. As the pressure increases with depth in a glacier from the weight of the ice above, the pressure melting point of ice decreases within bounds, as shown in the diagram. The level where ice can start melting is where the pressure melting point equals the actual temperature.[2] In static equilibrium conditions, this would be the highest level where water can exist in a glacier. It would also be the level of the base of an ice shelf, or the ice-water interface of a subglacial lake.[3]
References
- Sharp, Robert Phillip (1988). Living Ice: Understanding Glaciers and Glaciation. Cambridge University Press. pp. 27. ISBN 0-521-33009-2.
https://en.wikipedia.org/wiki/Pressure_melting_point
An ice shelf is a large floating platform of ice that forms where a glacier or ice sheet flows down to a coastline and onto the ocean surface. Ice shelves are only found in Antarctica, Greenland, Northern Canada, and the Russian Arctic. The boundary between the floating ice shelf and the anchor ice (resting on bedrock) that feeds it is the grounding line. The thickness of ice shelves can range from about 100 m (330 ft) to 1,000 m (3,300 ft).
https://en.wikipedia.org/wiki/Ice_shelf
| Part of a series on |
| Water salinity |
|---|
 |
| Salinity levels |
|
Fresh water (< 0.05%) Brackish water (0.05–3%) Saline water (3–5%) Brine (> 5% up to 26%–28% max) |
| Bodies of water |
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity
of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram
(roughly one liter by volume) of seawater has approximately 35 grams
(1.2 oz) of dissolved salts (predominantly sodium (Na+
) and chloride (Cl−
) ions). The average density at the surface is 1.025 kg/L. Seawater is denser than both fresh water
and pure water (density 1.0 kg/L at 4 °C (39 °F)) because the dissolved
salts increase the mass by a larger proportion than the volume. The
freezing point of seawater decreases as salt concentration increases. At
typical salinity, it freezes at about −2 °C (28 °F).[1] The coldest seawater still in the liquid state ever recorded was found in 2010, in a stream under an Antarctic glacier: the measured temperature was −2.6 °C (27.3 °F).[2]
Seawater pH is typically limited to a range between 7.5 and 8.4.[3] However, there is no universally accepted reference pH-scale for seawater and the difference between measurements based on different reference scales may be up to 0.14 units.[4]
https://en.wikipedia.org/wiki/Seawater
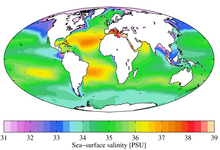
Annual mean sea surface salinity expressed in the Practical Salinity Scale for the World Ocean. Data from the World Ocean Atlas[5]
https://en.wikipedia.org/wiki/Seawater
| Part of a series on |
| Water salinity |
|---|
 |
| Salinity levels |
|
Fresh water (< 0.05%) Brackish water (0.05–3%) Saline water (3–5%) Brine (> 5% up to 26%–28% max) |
| Bodies of water |
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity
of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram
(roughly one liter by volume) of seawater has approximately 35 grams
(1.2 oz) of dissolved salts (predominantly sodium (Na+
) and chloride (Cl−
) ions). The average density at the surface is 1.025 kg/L. Seawater is denser than both fresh water
and pure water (density 1.0 kg/L at 4 °C (39 °F)) because the dissolved
salts increase the mass by a larger proportion than the volume. The
freezing point of seawater decreases as salt concentration increases. At
typical salinity, it freezes at about −2 °C (28 °F).[1] The coldest seawater still in the liquid state ever recorded was found in 2010, in a stream under an Antarctic glacier: the measured temperature was −2.6 °C (27.3 °F).[2]
Seawater pH is typically limited to a range between 7.5 and 8.4.[3] However, there is no universally accepted reference pH-scale for seawater and the difference between measurements based on different reference scales may be up to 0.14 units.[4]
Properties
Salinity
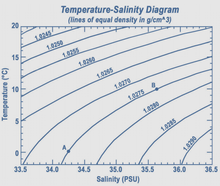
Although the vast majority of seawater has a salinity of between 31 and 38 g/kg, that is 3.1–3.8%, seawater is not uniformly saline throughout the world. Where mixing occurs with freshwater runoff from river mouths, near melting glaciers or vast amounts of precipitation (e.g. Monsoon), seawater can be substantially less saline. The most saline open sea is the Red Sea, where high rates of evaporation, low precipitation and low river run-off, and confined circulation result in unusually salty water. The salinity in isolated bodies of water can be considerably greater still – about ten times higher in the case of the Dead Sea. Historically, several salinity scales were used to approximate the absolute salinity of seawater. A popular scale was the "Practical Salinity Scale" where salinity was measured in "practical salinity units (PSU)". The current standard for salinity is the "Reference Salinity" scale [6] with the salinity expressed in units of "g/kg".
Density
The density of surface seawater ranges from about 1020 to 1029 kg/m3, depending on the temperature and salinity. At a temperature of 25 °C, the salinity of 35 g/kg and 1 atm pressure, the density of seawater is 1023.6 kg/m3.[7][8] Deep in the ocean, under high pressure, seawater can reach a density of 1050 kg/m3 or higher. The density of seawater also changes with salinity. Brines generated by seawater desalination plants can have salinities up to 120 g/kg. The density of typical seawater brine of 120 g/kg salinity at 25 °C and atmospheric pressure is 1088 kg/m3.[7][8]
pH value
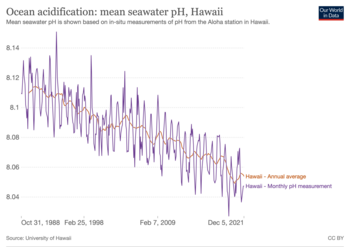
The pH value at the surface of oceans in pre-industrial time (before 1850) was around 8.2.[9] Since then, it has been decreasing due to a human-caused process called ocean acidification that is related to carbon dioxide emissions: Between 1950 and 2020, the average pH of the ocean surface fell from approximately 8.15 to 8.05.[10]
The pH value of seawater is naturally as low as 7.8 in deep ocean waters as a result of degradation of organic matter in these waters.[11] It can be as high as 8.4 in surface waters in areas of high biological productivity.[12]
Measurement of pH is complicated by the chemical properties of seawater, and several distinct pH scales exist in chemical oceanography.[13] There is no universally accepted reference pH-scale for seawater and the difference between measurements based on different reference scales may be up to 0.14 units.[4]
Chemical composition
Seawater contains more dissolved ions than all types of freshwater.[14] However, the ratios of solutes differ dramatically. For instance, although seawater contains about 2.8 times more bicarbonate than river water, the percentage of bicarbonate in seawater as a ratio of all dissolved ions is far lower than in river water. Bicarbonate ions constitute 48% of river water solutes but only 0.14% for seawater.[14][15] Differences like these are due to the varying residence times of seawater solutes; sodium and chloride have very long residence times, while calcium (vital for carbonate formation) tends to precipitate much more quickly.[15] The most abundant dissolved ions in seawater are sodium, chloride, magnesium, sulfate and calcium.[16] Its osmolarity is about 1000 mOsm/L.[17]
Small amounts of other substances are found, including amino acids at concentrations of up to 2 micrograms of nitrogen atoms per liter,[18] which are thought to have played a key role in the origin of life.
55%, Na+
30.6%, SO2−
4 7.7%, Mg2+
3.7%, Ca2+
1.2%, K+
1.1%, Other 0.7%. Note that the diagram is only correct when in units of wt/wt, not wt/vol or vol/vol.
| Element | Percent by mass |
|---|---|
| Oxygen | 85.84 |
| Hydrogen | 10.82 |
| Chlorine | 1.94 |
| Sodium | 1.08 |
| Magnesium | 0.1292 |
| Sulfur | 0.091 |
| Calcium | 0.04 |
| Potassium | 0.04 |
| Bromine | 0.0067 |
| Carbon | 0.0028 |
| Component | Concentration (mol/kg) |
|---|---|
| H 2O |
53.6 |
| Cl− |
0.546 |
| Na+ |
0.469 |
| Mg2+ |
0.0528 |
| SO2− 4 |
0.0282 |
| Ca2+ |
0.0103 |
| K+ |
0.0102 |
| CT | 0.00206 |
| Br− |
0.000844 |
| BT | 0.000416 |
| Sr2+ |
0.000091 |
| F− |
0.000068 |
Microbial components
Research in 1957 by the Scripps Institution of Oceanography sampled water in both pelagic and neritic locations in the Pacific Ocean. Direct microscopic counts and cultures were used, the direct counts in some cases showing up to 10 000 times that obtained from cultures. These differences were attributed to the occurrence of bacteria in aggregates, selective effects of the culture media, and the presence of inactive cells. A marked reduction in bacterial culture numbers was noted below the thermocline, but not by direct microscopic observation. Large numbers of spirilli-like forms were seen by microscope but not under cultivation. The disparity in numbers obtained by the two methods is well known in this and other fields.[20] In the 1990s, improved techniques of detection and identification of microbes by probing just small snippets of DNA, enabled researchers taking part in the Census of Marine Life to identify thousands of previously unknown microbes usually present only in small numbers. This revealed a far greater diversity than previously suspected, so that a litre of seawater may hold more than 20,000 species. Mitchell Sogin from the Marine Biological Laboratory feels that "the number of different kinds of bacteria in the oceans could eclipse five to 10 million."[21]
Bacteria are found at all depths in the water column, as well as in the sediments, some being aerobic, others anaerobic. Most are free-swimming, but some exist as symbionts within other organisms – examples of these being bioluminescent bacteria. Cyanobacteria played an important role in the evolution of ocean processes, enabling the development of stromatolites and oxygen in the atmosphere.
Some bacteria interact with diatoms, and form a critical link in the cycling of silicon in the ocean. One anaerobic species, Thiomargarita namibiensis, plays an important part in the breakdown of hydrogen sulfide eruptions from diatomaceous sediments off the Namibian coast, and generated by high rates of phytoplankton growth in the Benguela Current upwelling zone, eventually falling to the seafloor.
Bacteria-like Archaea surprised marine microbiologists by their survival and thriving in extreme environments, such as the hydrothermal vents on the ocean floor. Alkalotolerant marine bacteria such as Pseudomonas and Vibrio spp. survive in a pH range of 7.3 to 10.6, while some species will grow only at pH 10 to 10.6.[22] Archaea also exist in pelagic waters and may constitute as much as half the ocean's biomass, clearly playing an important part in oceanic processes.[23] In 2000 sediments from the ocean floor revealed a species of Archaea that breaks down methane, an important greenhouse gas and a major contributor to atmospheric warming.[24] Some bacteria break down the rocks of the sea floor, influencing seawater chemistry. Oil spills, and runoff containing human sewage and chemical pollutants have a marked effect on microbial life in the vicinity, as well as harbouring pathogens and toxins affecting all forms of marine life. The protist dinoflagellates may at certain times undergo population explosions called blooms or red tides, often after human-caused pollution. The process may produce metabolites known as biotoxins, which move along the ocean food chain, tainting higher-order animal consumers.
Pandoravirus salinus, a species of very large virus, with a genome much larger than that of any other virus species, was discovered in 2013. Like the other very large viruses Mimivirus and Megavirus, Pandoravirus infects amoebas, but its genome, containing 1.9 to 2.5 megabases of DNA, is twice as large as that of Megavirus, and it differs greatly from the other large viruses in appearance and in genome structure.
In 2013 researchers from Aberdeen University announced that they were starting a hunt for undiscovered chemicals in organisms that have evolved in deep sea trenches, hoping to find "the next generation" of antibiotics, anticipating an "antibiotic apocalypse" with a dearth of new infection-fighting drugs. The EU-funded research will start in the Atacama Trench and then move on to search trenches off New Zealand and Antarctica.[25]
The ocean has a long history of human waste disposal on the assumption that its vast size makes it capable of absorbing and diluting all noxious material.[26] While this may be true on a small scale, the large amounts of sewage routinely dumped has damaged many coastal ecosystems, and rendered them life-threatening. Pathogenic viruses and bacteria occur in such waters, such as Escherichia coli, Vibrio cholerae the cause of cholera, hepatitis A, hepatitis E and polio, along with protozoans causing giardiasis and cryptosporidiosis. These pathogens are routinely present in the ballast water of large vessels, and are widely spread when the ballast is discharged.[27]
Other parameters
The speed of sound in seawater is about 1,500 m/s (whereas the speed of sound is usually around 330 m/s in air at roughly 101.3 kPa pressure, 1 atmosphere), and varies with water temperature, salinity, and pressure. The thermal conductivity of seawater is 0.6 W/mK at 25 °C and a salinity of 35 g/kg.[28] The thermal conductivity decreases with increasing salinity and increases with increasing temperature.[29]
Origin and history
The water in the sea was thought to come from the Earth's volcanoes, starting 4 billion years ago, released by degassing from molten rock.[30]: 24–25 More recent work suggests much of the Earth's water may come from comets.[31]
Scientific theories behind the origins of sea salt started with Sir Edmond Halley in 1715, who proposed that salt and other minerals were carried into the sea by rivers after rainfall washed it out of the ground. Upon reaching the ocean, these salts concentrated as more salt arrived over time (see Hydrologic cycle). Halley noted that most lakes that don't have ocean outlets (such as the Dead Sea and the Caspian Sea, see endorheic basin), have high salt content. Halley termed this process "continental weathering".
Halley's theory was partly correct. In addition, sodium leached out of the ocean floor when the ocean formed. The presence of salt's other dominant ion, chloride, results from outgassing of chloride (as hydrochloric acid) with other gases from Earth's interior via volcanos and hydrothermal vents. The sodium and chloride ions subsequently became the most abundant constituents of sea salt.
Ocean salinity has been stable for billions of years, most likely as a consequence of a chemical/tectonic system which removes as much salt as is deposited; for instance, sodium and chloride sinks include evaporite deposits, pore-water burial, and reactions with seafloor basalts.[15]: 133
Human impacts
Climate change, rising levels of carbon dioxide in Earth's atmosphere, excess nutrients, and pollution in many forms are altering global oceanic geochemistry. Rates of change for some aspects greatly exceed those in the historical and recent geological record. Major trends include an increasing acidity, reduced subsurface oxygen in both near-shore and pelagic waters, rising coastal nitrogen levels, and widespread increases in mercury and persistent organic pollutants. Most of these perturbations are tied either directly or indirectly to human fossil fuel combustion, fertilizer, and industrial activity. Concentrations are projected to grow in coming decades, with negative impacts on ocean biota and other marine resources.[32]
One of the most striking features of this is ocean acidification, resulting from increased CO2 uptake of the oceans related to higher atmospheric concentration of CO2 and higher temperatures,[33] because it severely affects coral reefs, mollusks, echinoderms and crustaceans (see coral bleaching).
Human consumption
Accidentally consuming small quantities of clean seawater is not harmful, especially if the seawater is taken along with a larger quantity of fresh water. However, drinking seawater to maintain hydration is counterproductive; more water must be excreted to eliminate the salt (via urine) than the amount of water obtained from the seawater itself.[34] In normal circumstances, it would be considered ill-advised to consume large amounts of unfiltered seawater.
The renal system actively regulates the levels of sodium and chloride in the blood within a very narrow range around 9 g/L (0.9% by mass).
In most open waters concentrations vary somewhat around typical values of about 3.5%, far higher than the body can tolerate and most beyond what the kidney can process. A point frequently overlooked in claims that the kidney can excrete NaCl in Baltic concentrations of 2% (in arguments to the contrary) is that the gut cannot absorb water at such concentrations, so that there is no benefit in drinking such water. The salinity of Baltic surface water, however, is never 2%. It is 0.9% or less, and thus never higher than that of bodily fluids. Drinking seawater temporarily increases blood's NaCl concentration. This signals the kidney to excrete sodium, but seawater's sodium concentration is above the kidney's maximum concentrating ability. Eventually the blood's sodium concentration rises to toxic levels, removing water from cells and interfering with nerve conduction, ultimately producing fatal seizure and cardiac arrhythmia.[citation needed]
Survival manuals consistently advise against drinking seawater.[35] A summary of 163 life raft voyages estimated the risk of death at 39% for those who drank seawater, compared to 3% for those who did not. The effect of seawater intake on rats confirmed the negative effects of drinking seawater when dehydrated.[36]
The temptation to drink seawater was greatest for sailors who had expended their supply of fresh water and were unable to capture enough rainwater for drinking. This frustration was described famously by a line from Samuel Taylor Coleridge's The Rime of the Ancient Mariner:
Water, water, everywhere,
And all the boards did shrink;
Water, water, everywhere,
Nor any drop to drink.
Although humans cannot survive on seawater, some people claim that up to two cups a day, mixed with fresh water in a 2:3 ratio, produces no ill effect. The French physician Alain Bombard survived an ocean crossing in a small Zodiak rubber boat using mainly raw fish meat, which contains about 40% water (like most living tissues), as well as small amounts of seawater and other provisions harvested from the ocean. His findings were challenged, but an alternative explanation was not given. In his 1948 book The Kon-Tiki Expedition, Thor Heyerdahl reported drinking seawater mixed with fresh in a 2:3 ratio during the 1947 expedition.[37] A few years later, another adventurer, William Willis, claimed to have drunk two cups of seawater and one cup of fresh per day for 70 days without ill effect when he lost part of his water supply.[38]
During the 18th century, Richard Russell advocated the medical use of this practice in the UK,[39] and René Quinton expanded the advocation of this practice to other countries, notably France, in the 20th century. Currently, it is widely practiced in Nicaragua and other countries, supposedly taking advantage of the latest medical discoveries.[40][41]
Most oceangoing vessels desalinate potable water from seawater using processes such as vacuum distillation or multi-stage flash distillation in an evaporator, or, more recently, reverse osmosis. These energy-intensive processes were not usually available during the Age of Sail. Larger sailing warships with large crews, such as Nelson's HMS Victory, were fitted with distilling apparatus in their galleys.[42] Animals such as fish, whales, sea turtles, and seabirds, such as penguins and albatrosses, have adapted to living in a high-saline habitat. For example, sea turtles and saltwater crocodiles remove excess salt from their bodies through their tear ducts.[43]
Mineral extraction
Minerals have been extracted from seawater since ancient times. Currently the four most concentrated metals – Na, Mg, Ca and K – are commercially extracted from seawater.[44] During 2015 in the US 63% of magnesium production came from seawater and brines.[45] Bromine is also produced from seawater in China and Japan.[46] Lithium extraction from seawater was tried in the 1970s, but the tests were soon abandoned. The idea of extracting uranium from seawater has been considered at least from the 1960s, but only a few grams of uranium were extracted in Japan in the late 1990s.[47] The main issue is not one of technological feasibility but that current prices on the uranium market for uranium from other sources are about three to five times lower than the lowest price achieved by seawater extraction.[48][49] Similar issues hamper the use of reprocessed uranium and are often brought forth against nuclear reprocessing and the manufacturing of MOX fuel as economically unviable.
Standard
ASTM International has an international standard for artificial seawater: ASTM D1141-98 (Original Standard ASTM D1141-52). It is used in many research testing labs as a reproducible solution for seawater such as tests on corrosion, oil contamination, and detergency evaluation.[50]
See also
- Brine – Concentrated solution of salt in water
- Brine mining – Extracting materials from saltwater
- Brackish water – Water with salinity between freshwater and seawater
- Fresh water – Naturally occurring water with low amounts of dissolved salts
- Ocean color – Explanation of the color of oceans and ocean color remote sensing
- Saline water – Water that contains a high concentration of dissolved salts
- Sea ice – Ice formed from frozen seawater
- Seawater pH – Measure of the level of acidity or basicity of an aqueous solution
- Surface tension of seawater – Tendency of a liquid surface to shrink to reduce surface area
- Thalassotherapy
- Thermohaline circulation – Part of large-scale ocean circulation
- CORA dataset – free global oceanographic temperature and salinity dataset global ocean salinity
References
- "ASTM D1141-98(2013)". ASTM. Retrieved 17 August 2013.
External links
Tables
- Tables and software for thermophysical properties of seawater, MIT
- G. W. C Kaye, T. H. Laby (1995). "Physical properties of sea water". Tables of physical and chemical constants (16th ed.). Archived from the original on 8 May 2019.
https://en.wikipedia.org/wiki/Seawater
Ocean acidification is the decrease in the pH of the Earth's ocean. Between 1950 and 2020, the average pH of the ocean surface fell from approximately 8.15 to 8.05.[2] Carbon dioxide emissions from human activities are the primary cause of ocean acidification, with atmospheric carbon dioxide (CO2) levels exceeding 410 ppm (in 2020). CO2 from the atmosphere is absorbed by the oceans. This produces carbonic acid (H2CO3) which dissociates into a bicarbonate ion (HCO−3) and a hydrogen ion (H+). The presence of free hydrogen ions (H+) lowers the pH of the ocean, increasing acidity (this does not mean that seawater is acidic yet; it is still alkaline, with a pH higher than 8). Marine calcifying organisms, such as mollusks and corals, are especially vulnerable because they rely on calcium carbonate to build shells and skeletons.[3]
https://en.wikipedia.org/wiki/Ocean_acidification
| Part of a series on |
| Water salinity |
|---|
 |
| Salinity levels |
|
Fresh water (< 0.05%) Brackish water (0.05–3%) Saline water (3–5%) Brine (> 5% up to 26%–28% max) |
| Bodies of water |
Brine (or Briny water) is a high-concentration solution of salt (typically sodium chloride or calcium chloride) in water. In diverse contexts, brine may refer to the salt solutions ranging from about 3.5% (a typical concentration of seawater, on the lower end of that of solutions used for brining foods) up to about 26% (a typical saturated solution, depending on temperature). Brine forms naturally due to evaporation of ground saline water but it is also generated in the mining of sodium chloride.[1] Brine is used for food processing and cooking (pickling and brining), for de-icing of roads and other structures, and in a number of technological processes. It is also a by-product of many industrial processes, such as desalination, so it requires wastewater treatment for proper disposal or further utilization (fresh water recovery).[2]
https://en.wikipedia.org/wiki/Brine
This section needs additional citations for verification. (November 2022) |
Fresh water or freshwater is any naturally occurring liquid or frozen water containing low concentrations of dissolved salts and other total dissolved solids. Although the term specifically excludes seawater and brackish water, it does include non-salty mineral-rich waters such as chalybeate springs. Fresh water may encompass frozen and meltwater in ice sheets, ice caps, glaciers, snowfields and icebergs, natural precipitations such as rainfall, snowfall, hail/sleet and graupel, and surface runoffs that form inland bodies of water such as wetlands, ponds, lakes, rivers, streams, as well as groundwater contained in aquifers, subterranean rivers and lakes. Fresh water is the water resource that is of the most and immediate use to humans.
Water is critical to the survival of all living organisms. Many organisms can thrive on salt water, but the great majority of higher plants and most insects, amphibians, reptiles, mammals and birds need fresh water to survive.
Fresh water is not always potable water, that is, water safe to drink by humans. Much of the earth's fresh water (on the surface and groundwater) is to a substantial degree unsuitable for human consumption without some treatment. Fresh water can easily become polluted by human activities or due to naturally occurring processes, such as erosion. Fresh water makes up less than 3% of the world's water resources, and just 1% of that is readily available. Just 3% of it is extracted for human consumption. Agriculture uses roughly two thirds of all fresh water abstracted from the environment.[1][2][3]
Fresh water is a renewable and variable, but finite natural resource. Fresh water is replenished through the process of the natural water cycle, in which water from seas, lakes, forests, land, rivers and reservoirs evaporates, forms clouds, and returns inland as precipitation.[4] Locally, however, if more fresh water is consumed through human activities than is naturally restored, this may result in reduced fresh water availability (or water scarcity) from surface and underground sources and can cause serious damage to surrounding and associated environments. Water pollution also reduces the availability of fresh water.
Definitions
| Part of a series on |
| Water salinity |
|---|
 |
| Salinity levels |
|
Fresh water (< 0.05%) Brackish water (0.05–3%) Saline water (3–5%) Brine (> 5% up to 26%–28% max) |
| Bodies of water |
Numerical definition
Fresh water can be defined as water with less than 500 parts per million (ppm) of dissolved salts.[5]
Other sources give higher upper salinity limits for fresh water, e.g. 1,000 ppm[6] or 3,000 ppm.[7]
Systems
Fresh water habitats are classified as either lentic systems, which are the stillwaters including ponds, lakes, swamps and mires; lotic which are running-water systems; or groundwaters which flow in rocks and aquifers. There is, in addition, a zone which bridges between groundwater and lotic systems, which is the hyporheic zone, which underlies many larger rivers and can contain substantially more water than is seen in the open channel. It may also be in direct contact with the underlying underground water.
Sources
The original source of almost all fresh water is precipitation from the atmosphere, in the form of mist, rain and snow. Fresh water falling as mist, rain or snow contains materials dissolved from the atmosphere and material from the sea and land over which the rain bearing clouds have traveled. The precipitation leads eventually to the formation of water bodies that humans can use as sources of freshwater: ponds, lakes, rainfall, rivers, streams, and groundwater contained in underground aquifers.
In coastal areas fresh water may contain significant concentrations of salts derived from the sea if windy conditions have lifted drops of seawater into the rain-bearing clouds. This can give rise to elevated concentrations of sodium, chloride, magnesium and sulfate as well as many other compounds in smaller concentrations.
In desert areas, or areas with impoverished or dusty soils, rain-bearing winds can pick up sand and dust and this can be deposited elsewhere in precipitation and causing the freshwater flow to be measurably contaminated both by insoluble solids but also by the soluble components of those soils. Significant quantities of iron may be transported in this way including the well-documented transfer of iron-rich rainfall falling in Brazil derived from sand-storms in the Sahara in north Africa.[citation needed][8]
Water distribution
Saline water in oceans, seas and saline groundwater make up about 97% of all the water on Earth. Only 2.5–2.75% is fresh water, including 1.75–2% frozen in glaciers, ice and snow, 0.5–0.75% as fresh groundwater and soil moisture, and less than 0.01% of it as surface water in lakes, swamps and rivers.[10][11] Freshwater lakes contain about 87% of this fresh surface water, including 29% in the African Great Lakes, 22% in Lake Baikal in Russia, 21% in the North American Great Lakes, and 14% in other lakes. Swamps have most of the balance with only a small amount in rivers, most notably the Amazon River. The atmosphere contains 0.04% water.[12] In areas with no fresh water on the ground surface, fresh water derived from precipitation may, because of its lower density, overlie saline ground water in lenses or layers. Most of the world's fresh water is frozen in ice sheets. Many areas have very little fresh water, such as deserts.
Freshwater ecosystems
Water is a critical issue for the survival of all living organisms. Some can use salt water but many organisms including the great majority of higher plants and most mammals must have access to fresh water to live. Some terrestrial mammals, especially desert rodents, appear to survive without drinking, but they do generate water through the metabolism of cereal seeds, and they also have mechanisms to conserve water to the maximum degree.
Freshwater ecosystems are a subset of Earth's aquatic ecosystems. They include lakes, ponds, rivers, streams, springs, bogs, and wetlands.[13] They can be contrasted with marine ecosystems, which have a larger salt content. Freshwater habitats can be classified by different factors, including temperature, light penetration, nutrients, and vegetation. There are three basic types of freshwater ecosystems: Lentic (slow moving water, including pools, ponds, and lakes), lotic (faster moving water, for example streams and rivers) and wetlands (areas where the soil is saturated or inundated for at least part of the time).[14][13] Freshwater ecosystems contain 41% of the world's known fish species.[15]
Freshwater ecosystems have undergone substantial transformations over time, which has impacted various characteristics of the ecosystems.[16] Original attempts to understand and monitor freshwater ecosystems were spurred on by threats to human health (for example cholera outbreaks due to sewage contamination).[17] Early monitoring focused on chemical indicators, then bacteria, and finally algae, fungi and protozoa. A new type of monitoring involves quantifying differing groups of organisms (macroinvertebrates, macrophytes and fish) and measuring the stream conditions associated with them.[18]Challenges
The increase in the world population and the increase in per capita water use puts increasing strains on the finite resources availability of clean fresh water. The response by freshwater ecosystems to a changing climate can be described in terms of three interrelated components: water quality, water quantity or volume, and water timing. A change in one often leads to shifts in the others as well.[19]
Limited resource
Water scarcity (closely related to water stress or water crisis) is the lack of fresh water resources to meet the standard water demand. There are two types of water scarcity: physical water scarcity and economic water scarcity.[20]: 560 Physical water scarcity is where there is not enough water to meet all demands, including that needed for ecosystems to function. Arid areas for example Central and West Asia, and North Africa often suffer from physical water scarcity.[21] On the other hand, economic water scarcity is the result of a lack of investment in infrastructure or technology to draw water from rivers, aquifers, or other water sources, or insufficient human capacity to meet the demand for water.[20]: 560 Much of Sub-Saharan Africa has economic water scarcity.[22]: 11
There is enough freshwater available globally and averaged over the year to meet demand. As such, water scarcity is caused by a mismatch between when and where people need water, and when and where it is available.[23] The main driving forces for the rising global demand for water are the increasing world population, improving living standards, changing consumption patterns (for example a dietary shift toward more animal products),[24] and expansion of irrigated agriculture.[25][26] Climate change (including droughts or floods), deforestation, increased water pollution and wasteful use of water can also cause insufficient water supply.[27] Scarcity varies over time as a result of natural hydrological variability, but varies even more so as a function of prevailing economic policy, planning and management approaches.Minimum streamflow
An important concern for hydrological ecosystems is securing minimum streamflow, especially preserving and restoring instream water allocations.[28] Fresh water is an important natural resource necessary for the survival of all ecosystems.
Water pollution
Water pollution (or aquatic pollution) is the contamination of water bodies, usually as a result of human activities, so that it negatively affects its uses.[29]: 6 Water bodies include lakes, rivers, oceans, aquifers, reservoirs and groundwater. Water pollution results when contaminants mix with these water bodies. Contaminants can come from one of four main sources: sewage discharges, industrial activities, agricultural activities, and urban runoff including stormwater.[30] Water pollution is either surface water pollution or groundwater pollution. This form of pollution can lead to many problems, such as the degradation of aquatic ecosystems or spreading water-borne diseases when people use polluted water for drinking or irrigation.[31] Another problem is that water pollution reduces the ecosystem services (such as providing drinking water) that the water resource would otherwise provide.
Sources of water pollution are either point sources or non-point sources. Point sources have one identifiable cause, such as a storm drain, a wastewater treatment plant or an oil spill. Non-point sources are more diffuse, such as agricultural runoff.[32] Pollution is the result of the cumulative effect over time. Pollution may take the form of toxic substances (e.g., oil, metals, plastics, pesticides, persistent organic pollutants, industrial waste products), stressful conditions (e.g., changes of pH, hypoxia or anoxia, increased temperatures, excessive turbidity, changes of salinity), or the introduction of pathogenic organisms. Contaminants may include organic and inorganic substances. A common cause of thermal pollution is the use of water as a coolant by power plants and industrial manufacturers.Society and culture
Human uses
Uses of water include agricultural, industrial, household, recreational and environmental activities.
Global goals for conservation
The Sustainable Development Goals are a collection of 17 interlinked global goals designed to be a "blueprint to achieve a better and more sustainable future for all".[33] Targets on freshwater conservation are included in SDG 6 (Clean water and sanitation) and SDG 15 (Life on land). For example, Target 6.4 is formulated as "By 2030, substantially increase water-use efficiency across all sectors and ensure sustainable withdrawals and supply of freshwater to address water scarcity and substantially reduce the number of people suffering from water scarcity."[33] Another target, Target 15.1, is: "By 2020, ensure the conservation, restoration and sustainable use of terrestrial and inland freshwater ecosystems and their services, in particular forests, wetlands, mountains and drylands, in line with obligations under international agreements."[33]
See also
- Limnology – Science of inland aquatic ecosystems
- Properties of water – Physical and chemical properties of pure water
Notes
- Only 3% of the Earth's water is fresh water. Most of it is in icecaps and glaciers (69%) and groundwater (30%), while all lakes, rivers and swamps combined only account for a small fraction (0.3%) of the Earth's total freshwater reserves.[citation needed]
Subnotes
- The entire block comprises 1 million tiny cubes.
References
- United Nations (2017) Resolution adopted by the General Assembly on 6 July 2017, Work of the Statistical Commission pertaining to the 2030 Agenda for Sustainable Development (A/RES/71/313 Archived 23 October 2020 at the Wayback Machine)
External links
- The World Bank's work and publications on water resources
- U.S. Geological Survey Archived 6 August 2012 at the Wayback Machine
- Fresh Water National Geographic Archived 27 November 2016 at the Wayback Machine
https://en.wikipedia.org/wiki/Fresh_water
| Air |
| ||||
|---|---|---|---|---|---|
| Energy | |||||
| Land | |||||
| Life | |||||
| Water |
| ||||
| Related |
| ||||
https://en.wikipedia.org/wiki/Fresh_water
https://en.wikipedia.org/wiki/Global_commons
https://en.wikipedia.org/wiki/Enclosure
https://en.wikipedia.org/wiki/Common_land
https://en.wikipedia.org/wiki/Tragedy_of_the_commons
https://en.wikipedia.org/wiki/Commons
https://en.wikipedia.org/wiki/Natural_resource_economics
https://en.wikipedia.org/wiki/Overexploitation
https://en.wikipedia.org/wiki/Natural_capital_accounting
https://en.wikipedia.org/wiki/Natural_heritage
https://en.wikipedia.org/wiki/Nature_reserve
https://en.wikipedia.org/wiki/Systems_ecology
https://en.wikipedia.org/wiki/Wilderness
https://en.wikipedia.org/wiki/Resource_war#Conflict_resources
https://en.wikipedia.org/wiki/Resource_curse
https://en.wikipedia.org/wiki/Resource_depletion
https://en.wikipedia.org/wiki/Natural_resource#Extraction
https://en.wikipedia.org/wiki/Non-renewable_resource
https://en.wikipedia.org/wiki/Groundwater
https://en.wikipedia.org/wiki/Aquifer_storage_and_recovery
https://en.wikipedia.org/wiki/Iceberg
https://en.wikipedia.org/wiki/Irrigation
https://en.wikipedia.org/wiki/Rainwater_harvesting
https://en.wikipedia.org/wiki/Stormwater
https://en.wikipedia.org/wiki/Surface_water
https://en.wikipedia.org/wiki/Sewage
https://en.wikipedia.org/wiki/Reclaimed_water
https://en.wikipedia.org/wiki/Drainage_basin
https://en.wikipedia.org/wiki/Water_resources
https://en.wikipedia.org/wiki/Wood
https://en.wikipedia.org/wiki/Seed_bank
https://en.wikipedia.org/wiki/Wildlife
https://en.wikipedia.org/wiki/Wildlife_conservation
https://en.wikipedia.org/wiki/Plant
https://en.wikipedia.org/wiki/Meadow
https://en.wikipedia.org/wiki/Biosphere
https://en.wikipedia.org/wiki/Fishery
https://en.wikipedia.org/wiki/Forest
https://en.wikipedia.org/wiki/Gene_bank
https://en.wikipedia.org/wiki/Open_space_reserve
https://en.wikipedia.org/wiki/Mining
https://en.wikipedia.org/wiki/Ore
https://en.wikipedia.org/wiki/Industrial_mineral
https://en.wikipedia.org/wiki/Mineral
https://en.wikipedia.org/wiki/Arable_land
https://en.wikipedia.org/wiki/Land_degradation
https://en.wikipedia.org/wiki/Landscape
https://en.wikipedia.org/wiki/Soundscape
https://en.wikipedia.org/wiki/Sand_mining
https://en.wikipedia.org/wiki/Peak_phosphorus
https://en.wikipedia.org/wiki/Habitat_conservation
https://en.wikipedia.org/wiki/Soil_fertility
https://en.wikipedia.org/wiki/Soil_resilience
https://en.wikipedia.org/wiki/Soil_conservation
https://en.wikipedia.org/wiki/Soil
https://en.wikipedia.org/wiki/Land_use
https://en.wikipedia.org/wiki/Shade_(shadow)
https://en.wikipedia.org/wiki/Nuclear_power
https://en.wikipedia.org/wiki/Geothermal_energy
https://en.wikipedia.org/wiki/Peak_coal
https://en.wikipedia.org/wiki/Fossil_fuel
https://en.wikipedia.org/wiki/Fuel_gas
https://en.wikipedia.org/wiki/World_energy_resources
















No comments:
Post a Comment