Fungaes/mycotics/sporeluants/bacterial-sporulants-toxins-metabolite-virus/plasmid-gene-fragments-etc. (clostridium, mad cow disease, spongiform encephalitis, stephys (staph), etc.)
Endotoxins, intoxicants, toxins, poisons, genotoxing, etc.
mycosis, mycobacteriae, mycotic parasite, symbiotics/activators/etc., Mycosis fungoide, etc..
(Fungy eat decay matter sometime, secondary metabolite/product of metabolism/byproduct of metabolism/possibility of cell function-structure-cycle-etc./etc. of fungees is sometimes toxin (toxic or not; dependent toxicity or not; potent toxic or not; etc.) or toxic [or alternatively, sometimes not toxic, etc.]; some able to survive in nuclear particle environment, some may consume/utilize/transform/etc. straight nuclear particle, etc.; radioactive particle induce tissue death melt/smold/burn/decay/etc. (including force burns; knocked off axis; etc.) alteration to rate/environment/condition/ctrl/etc., tissue survival depend on rate match time limited (rate match inefficient - compensatory, synergism, harmonization, etc. - not perfect rate match but sufficient to sustain cellular struct-funct-etc. for time)
Fungal Leukemiaes (fungus, bacteria plasmid, viridae, hum genome vir activator, act of prev inf immun respo, nuclear exposure, alkylation agents, injury, protazoe/amoebaes, parasitics (esp blood and cellular intra parasitics))
Aspergillosis (fungy parasite facilitation - massive; Tumor lysis factor, with lead prior etc.)
Amoaebitic Disease of Brain
Protozoans - Nagleria Floweri
Miliary Tuberculosis
US-ASC0 - Radiation, Alkylation Agent, Biohazard/Disease Trans, genotox (mimetic)
Toxin Neurodegen
acute myeloblasic leuk
Purple book - Non-mycosis fungoides CD30, TNF, chromosomal transloc/gene deletion-seq-insert-modding w radioactive or potent-etc./etc., Nf-kB, plasmid, viral vector, etc.
HIV ET HLV HTV HTLV SIV
1. Particle
13. VOCs, inhabitable cells (no nucleus), bone cave, etc. (tiny little nutrient - blood; large viand nutrient - digestive; nitrogen - bladder; chlorine - st, protinated-fatulants, etc..
Human T-cell lymphotropic virus type 1 or human T-lymphotropic virus (HTLV-I), also called the adult T-cell lymphoma virus type 1, is a retrovirus of the human T-lymphotropic virus (HTLV) family that has been implicated in several kinds of diseases including very aggressive adult T-cell lymphoma (ATL), HTLV-I-associated myelopathy, uveitis, Strongyloides stercoralis hyper-infection and some other diseases. It is thought that about 1–5% of infected persons develop cancer as a result of the infection with HTLV-I over their lifetimes.[1]
Adult T-cell lymphoma (ATL) was discovered in 1977 in Japan. The symptoms of ATL were different from other lymphomas known at the time. It was suggested that ATL is caused by the infection of a retrovirus called ATLV.[2] Strikingly, ATLV had the transforming activity in vitro.[3] These studies established that the retrovirus infection is the cause of ATL. The retrovirus is now generally called HTLV-I because later studies proved that ATLV is the same as the firstly identified human retrovirus called HTLV discovered by Bernard Poiesz and Francis Ruscetti and their co-workers in the laboratory of Robert C. Gallo at the National Cancer Institute.[4] Infection with HTLV-I, like infection with other retroviruses, probably occurs for life. A patient infected with HTLV can be diagnosed when antibodies against HTLV-1 are detected in the serum.[1]
https://en.wikipedia.org/wiki/Human_T-lymphotropic_virus_1
Lipopolysaccharides (LPS), also known as endotoxins, are large molecules consisting of a lipid and a polysaccharide composed of O-antigen, outer core and inner core joined by a covalent bond; they are found in the outer membrane of Gram-negative bacteria. The term lipooligosaccharide ("LOS") is used to refer to a low-molecular-weight form of bacterial lipopolysaccharides.
Today, the term endotoxin is mostly used synonymously with LPS,[1] although there are a few endotoxins that are not related to LPS, such as the so-called delta endotoxin proteins secreted by Bacillus thuringiensis.
https://en.wikipedia.org/wiki/Lipopolysaccharide
Autoimmune lymphoproliferative syndrome (ALPS), is a form of lymphoproliferative disorder (LPDs). It affects lymphocyteapoptosis.[2]
It is a rare genetic disorder of abnormal lymphocyte survival caused by defective Fas mediated apoptosis.[3] Normally, after infectious insult, the immune system down-regulates by increasing Fas expression on activated B and T lymphocytes and Fas-ligand on activated T lymphocytes. Fas and Fas-ligand interact to trigger the caspase cascade, leading to cell apoptosis. Patients with ALPS have a defect in this apoptotic pathway, leading to chronic non-malignant lymphoproliferation, autoimmune disease, and secondary cancers.[4]
https://en.wikipedia.org/wiki/Autoimmune_lymphoproliferative_syndrome
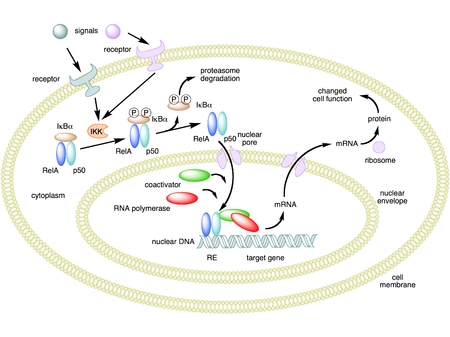
NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) is a protein complex that controls transcription of DNA, cytokine production and cell survival. NF-κB is found in almost all animal cell types and is involved in cellular responses to stimuli such as stress, cytokines, free radicals, heavy metals, ultraviolet irradiation, oxidized LDL, and bacterial or viral antigens.[1][2][3][5][6] NF-κB plays a key role in regulating the immune response to infection. Incorrect regulation of NF-κB has been linked to cancer, inflammatory and autoimmune diseases, septic shock, viral infection, and improper immune development. NF-κB has also been implicated in processes of synaptic plasticity and memory.[7][8][9][10][11][12]
https://en.wikipedia.org/wiki/NF-κB
Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system. They are single-pass membrane-spanning receptors usually expressed on sentinel cells such as macrophagesand dendritic cells, that recognize structurally conserved molecules derived from microbes. Once these microbes have breached physical barriers such as the skin or intestinal tract mucosa, they are recognized by TLRs, which activate immune cell responses. The TLRs include TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10, TLR11, TLR12, and TLR13, though the last three are not found in humans,[1] and there isn't a functional gene for TLR10 in mice. [2] TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are located on the cell membrane, whereas TLR3, TLR7, TLR8, and TLR9 are located in intracellular vesicles (because they are sensors of nucleic acids).[3]
TLRs received their name from their similarity to the protein coded by the toll gene identified in Drosophilain 1985 by Christiane Nüsslein-Volhard and Eric Wieschaus.[4]
https://en.wikipedia.org/wiki/Toll-like_receptor
In the field of molecular biology, nuclear receptors are a class of proteins found within cells that are responsible for sensing steroid and thyroid hormones and certain other molecules. In response, these receptors work with other proteins to regulate the expression of specific genes, thereby controlling the development, homeostasis, and metabolism of the organism.
Nuclear receptors have the ability to directly bind to DNA and regulate the expression of adjacent genes; hence these receptors are classified as transcription factors.[2][3] The regulation of gene expression by nuclear receptors generally only happens when a ligand—a molecule that affects the receptor's behavior—is present. More specifically, ligand binding to a nuclear receptor results in a conformational change in the receptor, which, in turn, activates the receptor, resulting in up- or down-regulation of gene expression.
A unique property of nuclear receptors that differentiates them from other classes of receptorsis their ability to directly interact with and control the expression of genomic DNA. As a consequence, nuclear receptors play key roles in both embryonic development and adult homeostasis. As discussed below, nuclear receptors may be classified according to either mechanism[4][5] or homology.[6][7]
https://en.wikipedia.org/wiki/Nuclear_receptor
Volatile organic compounds are compounds that have a high vapor pressure and low water solubility. Many VOCs are human-made chemicals that are used and produced in the manufacture of paints, pharmaceuticals, and refrigerants. VOCs typically are industrial solvents, such as trichloroethylene; fuel oxygenates, such as methyl tert-butyl ether (MTBE); or by-products produced by chlorination in water treatment, such as chloroform. VOCs are often components of petroleum fuels, hydraulic fluids, paint thinners, and dry cleaning agents. VOCs are common ground-water contaminants.
https://www.epa.gov/indoor-air-quality-iaq/what-are-volatile-organic-compounds-vocs
Volatile organic compounds (VOC) are organic chemicals that have a high vapour pressure at room temperature. High vapor pressure correlates with a low boiling point, which relates to the number of the sample's molecules in the surrounding air, a trait known as volatility.[1]
VOCs are responsible for the odor of scents and perfumes as well as pollutants. VOCs play an important role in communication between animals and plants, e.g. attractants for pollinators,[2] protection from predation,[3] and even inter-plant interactions.[4] Some VOCs are dangerous to human health or cause harm to the environment. Anthropogenic VOCs are regulated by law, especially indoors, where concentrations are the highest. Most VOCs are not acutely toxic, but may have long-term chronic health effects.
https://en.wikipedia.org/wiki/Volatile_organic_compound
NF-κB is increasingly expressed with obesity and aging,[87] resulting in reduced levels of the anti-inflammatory, pro-autophagy, anti-insulin resistanceprotein sirtuin 1. NF-κB increases the levels of the microRNA miR-34a (which inhibits nicotinamide adenine dinucleotide NAD synthesis) by binding to its promoter region.[88] resulting in lower levels of sirtuin 1.
NF-κB and interleukin 1 alpha mutually induce each other in senescent cells in a positive feedback loop causing the production of senescence-associated secretory phenotype (SASP) factors.[89]
Aberrant activation of NF-κB is frequently observed in many cancers. Moreover, suppression of NF-κB limits the proliferation of cancer cells. In addition, NF-κB is a key player in the inflammatory response. Hence methods of inhibiting NF-κB signaling has potential therapeutic application in cancer and inflammatory diseases.[101][102]
Both the canonical and non-canonical NF-κB pathways require proteasomal degradation of regulatory pathway components for NF-κB signalling to occur. The proteosome inhibitor Bortezomib broadly blocks this activity and is approved for treatment of NF-κB driven Mantle Cell Lymphoma and Multiple Myeloma.[103][104]
The discovery that activation of NF-κB nuclear translocation can be separated from the elevation of oxidant stress[105] gives a promising avenue of development for strategies targeting NF-κB inhibition.
The drug denosumab acts to raise bone mineral density and reduce fracture rates in many patient sub-groups by inhibiting RANKL. RANKL acts through its receptor RANK, which in turn promotes NF-κB,[106] RANKL normally works by enabling the differentiation of osteoclasts from monocytes.
Disulfiram, olmesartan and dithiocarbamates can inhibit the nuclear factor-κB (NF-κB) signaling cascade.[107] Effort to develop direct NF-κB inhibitor has emerged with compounds such as (-)-DHMEQ, PBS-1086, IT-603 and IT-901.[108][109][110] (-)-DHMEQ and PBS-1086 are irreversible binder to NF-κB while IT-603 and IT-901 are reversible binder. DHMEQ covalently binds to Cys 38 of p65.[111]
Anatabine's antiinflammatory effects are claimed to result from modulation of NF-κB activity.[112] However the studies purporting its benefit use abnormally high doses in the millimolar range (similar to the extracellular potassium concentration), which are unlikely to be achieved in humans.
BAY 11-7082 has also been identified as a drug that can inhibit the NF-κB signaling cascade. It is capable of preventing the phosphorylation of IKK-α in an irreversible manner such that there is down regulation of NF-κB activation.[113]
It has been shown that administration of BAY 11-7082 rescued renal functionality in diabetic-induced Sprague-Dawley rats by suppressing NF-κB regulated oxidative stress.[114]
Research has shown that the N-acylethanolamine, palmitoylethanolamide is capable of PPAR-mediated inhibition of NF-κB.[115]
The biological target of iguratimod, a drug marketed to treat rheumatoid arthritis in Japan and China, was unknown as of 2015, but the primary mechanism of action appeared to be preventing NF-κB activation.[116]
https://en.wikipedia.org/wiki/NF-κB
Plasmodium falciparum is a unicellular protozoan parasite of humans, and the deadliest species of Plasmodium that causes malaria in humans.[2] The parasite is transmitted through the bite of a female Anopheles mosquito and causes the disease's most dangerous form, falciparum malaria. It is responsible for around 50% of all malaria cases.[3][4] P. falciparum is therefore regarded as the deadliest parasite in humans. It is also associated with the development of blood cancer (Burkitt's lymphoma) and is classified as Group 2A carcinogen.
The species originated from the malarial parasite Laverania found in gorillas, around 10,000 years ago.[5]Alphonse Laveran was the first to identify the parasite in 1880, and named it Oscillaria malariae. Ronald Rossdiscovered its transmission by mosquito in 1897. Giovanni Battista Grassi elucidated the complete transmission from a female anopheline mosquito to humans in 1898. In 1897, William H. Welch created the name Plasmodium falciparum, which ICZN formally adopted in 1954. P. falciparum assumes several different forms during its life cycle. The human-infective stage are sporozoites from the salivary gland of a mosquito. The sporozoites grow and multiply in the liver to become merozoites. These merozoites invade the erythrocytes(RBCs) to form trophozoites, schizonts and gametocytes, during which the symptoms of malaria are produced. In the mosquito, the gametocytes undergo sexual reproduction to a zygote, which turns into ookinete. Ookinete forms oocytes from which sporozoites are formed.
https://en.wikipedia.org/wiki/Plasmodium_falciparum
Anatabine (uh-nat-uh-been,-bin) is one of the minor alkaloids found in plants in the family Solanaceae, which includes the tobacco plant and tomato. Commercial tobacco plants typically produce alkaloids at levels between 2% and 4% of total dry weight, with nicotine accounting for about 90% of the total alkaloid content, and the related compounds anabatine, nornicotine, and anabasine making up nearly all the rest.[1]These compounds are thought to be biologically active, and part of plants' natural defense system against insects.[1]
Anatabine has anti-inflammatory activity partly through inhibition of STAT3 phosphorylation in vitro and in vivo.[2]
https://en.wikipedia.org/wiki/Anatabine
Multiple myeloma (MM), also known as plasma cell myeloma and simply myeloma, is a cancerof plasma cells, a type of white blood cell that normally produces antibodies.[6] Often, no symptoms are noticed initially.[10] As it progresses, bone pain, anemia, kidney dysfunction, and infections may occur.[10] Complications may include amyloidosis.[3]
https://en.wikipedia.org/wiki/Multiple_myeloma
Y box binding protein 1 also known as Y-box transcription factor or nuclease-sensitive element-binding protein 1 is a protein that in humans is encoded by the YBX1 gene.[5]
https://en.wikipedia.org/wiki/Y_box_binding_protein_1
DNA-binding protein A is a protein that in humans is encoded by the CSDA gene.[5][6][7]
https://en.wikipedia.org/wiki/CSDA_(gene)
High-Mobility Group or HMG is a group of chromosomal proteins that are involved in the regulation of DNA-dependent processes such as transcription, replication, recombination, and DNA repair.[1]
https://en.wikipedia.org/wiki/High-mobility_group
Multiple myeloma may develop from monoclonal gammopathy of undetermined significance that progresses to smoldering myeloma.[13] The abnormal plasma cells produce abnormal antibodies, which can cause kidney problems and overly thick blood.[10] The plasma cells can also form a mass in the bone marrow or soft tissue.[10] When one tumor is present, it is called a plasmacytoma; more than one is called multiple myeloma.[10]Multiple myeloma is diagnosed based on blood or urine tests finding abnormal antibodies, bone marrow biopsy finding cancerous plasma cells, and medical imaging finding bone lesions.[6]Another common finding is high blood calcium levels.[6]
https://en.wikipedia.org/wiki/Multiple_myeloma
Nuclear factor of activated T-cells (NFAT) is a family of transcription factors shown to be important in immune response. One or more members of the NFAT family is expressed in most cells of the immune system. NFAT is also involved in the development of cardiac, skeletal muscle, and nervous systems. NFAT was first discovered as an activator for the transcription of IL-2 in T cells (as a regulator of T cell immune response) but has since been found to play an important role in regulating many more body systems.[1] NFAT transcription factors are involved in many normal body processes as well as in development of several diseases, such as inflammatory bowel diseases and several types of cancer. NFAT is also being investigated as a drug target for several different disorders.
https://en.wikipedia.org/wiki/NFAT
https://en.wikipedia.org/wiki/NF-κB
NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) is a protein complex that controls transcription of DNA, cytokine production and cell survival. NF-κB is found in almost all animal cell types and is involved in cellular responses to stimuli such as stress, cytokines, free radicals, heavy metals, ultraviolet irradiation, oxidized LDL, and bacterial or viral antigens.[1][2][3][5][6] NF-κB plays a key role in regulating the immune response to infection. Incorrect regulation of NF-κB has been linked to cancer, inflammatory and autoimmune diseases, septic shock, viral infection, and improper immune development. NF-κB has also been implicated in processes of synaptic plasticity and memory.[7][8][9][10][11][12]

No comments:
Post a Comment