| Australosphenida Temporal range:
| |
|---|---|

| |
| Jaw fragment of Ambondro mahabo | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Subclass: | Yinotheria |
| Infraclass: | Australosphenida Luo, Cifelli, & Kielan-Jaworowska, 2001 |
| Taxa | |
The Australosphenida are a clade of mammals, containing mammals with tribosphenic molars, known from the Jurassic to Mid-Cretaceous of Gondwana. They are generally thought to have acquired their tribosphenic molars independently from those of Tribosphenida. Fossils of australosphenidans have been found from the Jurassic of Madagascar and Argentina, and Cretaceous of Australia and Argentina. Monotremes have also been considered a part of this group in some studies, but this is disputed.
Taxonomy
This grouping includes the following taxa:
- †Henosferidae, including the genera Ambondro, Asfaltomylos, and Henosferus from the Jurassic of Argentina and Madagascar.
- †Ausktribosphenidae, including the genera Ausktribosphenos, Bishops from the Lower Cretaceous of Australia and Mid-Cretaceous of Argentina
- †Vincelestes, sometimes recovered as an australosphenidan (when not inversely considered a cladotherian).[1][2]
- †Tendagurutherium, also recently recovered as an australosphenidan.[1]
The clade Australosphenida was proposed by Luo et al. (2001, 2002) and was initially left unranked, as the authors do not apply the Linnaean hierarchy. In Benton (2005), it is ranked as a 'superdivision', i.e. one or two levels below the infraclass.
Evolution
The grouping embodies a hypothesis about the evolution of molar teeth in mammals. Living monotremes are toothless as adults, but the juvenile platypus, fossil monotremes and Ausktribosphenida all share a pattern of three molar cusps arranged in a triangle or V shape, which is known as the tribosphenic type of molar. Tribosphenic molars have long been held to characterize the subclass Theria (marsupials, placentals and their extinct relatives), while monotremes were thought to be related to fossil groups with a linear alignment of cusps: morganucodontids, docodonts, triconodonts and multituberculates, all of which were united with the monotremes into the 'subclass Prototheria'. Defined in this way, the 'Prototheria' is no longer recognised as a valid clade, since the linear cusp pattern is a primitive condition within Mammalia and cannot supply the shared derived character, which is required to establish a subgroup. Instead, the available evidence suggests that the monotremes descend from a Mesozoic radiation of tribosphenic mammals in the southern continents (hence the name Australosphenida, meaning 'southern wedges'), but this interpretation is highly controversial.
According to Luo et al., tribosphenic molars were evolved by the Australosphenida independently of the true Tribosphenida, or Boreosphenida (that is, the therians and their relatives) in the northern continents. Others contend that the ausktribosphenids (two families of the Australian Cretaceous tribosphenids) in fact belong to the placentals and were therefore true tribosphenids, but unrelated to the ancestry of the monotremes.[3]
Most recent phylogenetic studies, lump henosferids and aukstribosphenids alongside monotremes.[4][5] However in a 2022 review of montreme evolution noted that most primitive monotreme Teinolophos differed substantially from other non-monotreme Australosphenidans, having five molars as opposed to three in all other non-monotreme australosphenidans, and having non-tribosphenic molars, meaning that the two groups were likely unrelated.[6] Later, Flannery and coauthors suggested that the core grouping of australosphenidans (excluding monotremes) were actually stem-therians as members of Tribosphenida, with the group representing a paraphyletic grade, with Bishopsidae more closely related to Theria than to other australosphenidans.[7]
Notes
- Flannery, Timothy F.; Rich, Thomas H.; Vickers-Rich, Patricia; Veatch, E. Grace; Helgen, Kristofer M. (2022-11-01). "The Gondwanan Origin of Tribosphenida (Mammalia)". Alcheringa: An Australasian Journal of Palaeontology. 46 (3–4): 277–290. doi:10.1080/03115518.2022.2132288. ISSN 0311-5518. S2CID 253323862.
References
- Benton, Michael J. (2005). Vertebrate Palaeontology (3rd ed.). Oxford: Blackwell Publishing. ISBN 0-632-05637-1.
- Luo, Zhe-Xi; Cifelli, Richard L.; Kielan-Jaworowska, Zofia (2001). "Dual origin of tribosphenic mammals". Nature. 409 (6816): 53–57. doi:10.1038/35051023. PMID 11343108. S2CID 4342585.
- Luo, Zhe-Xi; Kielan-Jaworowska, Zofia; Cifelli, Richard L. (2002). "In quest for a phylogeny of Mesozoic mammals" (PDF). Acta Palaeontologica Polonica. 47 (1): 1–78.
https://en.wikipedia.org/wiki/Australosphenida
Monotreme
| Monotremes[1] Temporal range:
| |
|---|---|

| |
| Four of the five extant monotreme species: platypus (top-left), short-beaked echidna (top-right), western long-beaked echidna (bottom-left), and replica eastern long-beaked echidna (bottom-right) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Monotremata C.L. Bonaparte, 1837[2] |
| Subgroups | |
Monotremes (/ˈmɒnətriːmz/) are mammals of the order Monotremata. They are one of the three groups of living mammals, along with placentals (Eutheria), and marsupials (Metatheria). Monotremes are typified by structural differences in their brains, jaws, digestive tract, reproductive tract, and other body parts, compared to the more common mammalian types. In addition, they lay eggs rather than bearing live young, but, like all mammals, the female monotremes nurse their young with milk.
Monotremes have been considered members of Australosphenida, a clade that contains extinct mammals from the Jurassic and Cretaceous of Madagascar, South America, and Australia, though this is disputed.
The only surviving examples of monotremes are all indigenous to Australia and New Guinea, although they were also present in the Late Cretaceous and Paleocene of southern South America, indicating that they were also present in Antarctica, though remains have not been found there. The extant monotreme species are the platypus and four species of echidnas. There is currently some debate regarding monotreme taxonomy.
The name monotreme derives from the Greek words μονός (monós 'single') and τρῆμα (trêma 'hole'), referring to the cloaca.
General characteristics
Like other mammals, monotremes are endothermic with a high metabolic rate (though not as high as other mammals; see below); have hair on their bodies; produce milk through mammary glands to feed their young; have a single bone in their lower jaw; and have three middle-ear bones.
In common with reptiles and marsupials, monotremes lack the connective structure (corpus callosum) which in placental mammals is the primary communication route between the right and left brain hemispheres.[3] The anterior commissure does provide an alternate communication route between the two hemispheres, though, and in monotremes and marsupials it carries all the commissural fibers arising from the neocortex, whereas in placental mammals the anterior commissure carries only some of these fibers.[4]
Extant monotremes lack teeth as adults. Fossil forms and modern platypus young have a "tribosphenic" form of molars (with the occlusal surface formed by three cusps arranged in a triangle), which is one of the hallmarks of extant mammals. Some recent work suggests that monotremes acquired this form of molar independently of placental mammals and marsupials,[5] although this hypothesis remains disputed.[6] Tooth loss in modern monotremes might be related to their development of electrolocation.[7]
Monotreme jaws are constructed somewhat differently from those of other mammals, and the jaw opening muscle is different. As in all true mammals, the tiny bones that conduct sound to the inner ear are fully incorporated into the skull, rather than lying in the jaw as in non-mammal cynodonts and other premammalian synapsids; this feature, too, is now claimed to have evolved independently in monotremes and therians,[8] although, as with the analogous evolution of the tribosphenic molar, this hypothesis is disputed.[9][10] Nonetheless, findings on the extinct species Teinolophos confirm that suspended ear bones evolved independently among monotremes and therians.[11] The external opening of the ear still lies at the base of the jaw.
The sequencing of the platypus genome has also provided insight into the evolution of a number of monotreme traits, such as venom and electroreception, as well as showing some new unique features, such as monotremes possessing 5 pairs of sex chromosomes and that one of the X chromosomes resembles the Z chromosome of birds,[12] suggesting that the two sex chromosomes of marsupial and placental mammals evolved after the split from the monotreme lineage.[13] Additional reconstruction through shared genes in sex chromosomes supports this hypothesis of independent evolution.[14] This feature, along with some other genetic similarities with birds, such as shared genes related to egg-laying, is thought to provide some insight into the most recent common ancestor of the synapsid lineage leading to mammals and the sauropsid lineage leading to birds and modern reptiles, which are believed to have split about 315 million years ago during the Carboniferous.[15][16] The presence of vitellogenin genes (a protein necessary for egg shell formation) is shared with birds; the presence of this symplesiomorphy suggests that the common ancestor of monotremes, marsupials, and placental mammals was oviparous, and that this trait was retained in monotremes but lost in all other extant mammal groups. DNA analyses suggest that although this trait is shared and is synapomorphic with birds, platypuses are still mammals and that the common ancestor of extant mammals lactated.[17]
The monotremes also have extra bones in the shoulder girdle, including an interclavicle and coracoid, which are not found in other mammals. Monotremes retain a reptile-like gait, with legs on the sides of, rather than underneath, their bodies. The monotreme leg bears a spur in the ankle region; the spur is not functional in echidnas, but contains a powerful venom in the male platypus. This venom is derived from β-defensins, proteins that are present in mammals that create holes in viral and bacterial pathogens. Some reptile venom is also composed of different types of β-defensins, another trait shared with reptiles.[15] It is thought to be an ancient mammalian characteristic, as many non-monotreme archaic mammal groups also possess venomous spurs.[18]
Reproductive system
This section may be expanded with text translated from the corresponding article in French. (August 2014) Click [show] for important translation instructions. |
The key anatomical difference between monotremes and other mammals gives them their name; monotreme means “single opening” in Greek, referring to the single duct (the cloaca) for their urinary, defecatory, and reproductive systems. Like reptiles, monotremes have a single cloaca. Marsupials have a separate genital tract, whereas most placental mammalian females have separate openings for reproduction (the vagina), urination (the urethra), and defecation (the anus). In monotremes, only semen passes through the penis while urine is excreted through the male's cloaca.[19] The monotreme penis is similar to that of turtles and is covered by a preputial sac.[20][21]
Monotreme eggs are retained for some time within the mother and receive nutrients directly from her, generally hatching within 10 days after being laid — much shorter than the incubation period of sauropsid eggs.[22][23] Much like newborn marsupials (and perhaps all non-placental mammals[24]), newborn monotremes, called "puggles,"[25] are larval- and fetus-like and have relatively well-developed forelimbs that enable them to crawl around. In fact, because monotremes lack nipples, puggles crawl about more frequently than marsupial joeys in search of milk, this difference raising questions about the supposed developmental restrictions on marsupial forelimbs.[clarification needed][26]
Rather than through nipples, monotremes lactate from their mammary glands via openings in their skin. All five extant species show prolonged parental care of their young, with low rates of reproduction and relatively long life-spans.
Monotremes are also noteworthy in their zygotic development: Most mammalian zygotes go through holoblastic cleavage, where the ovum splits into multiple, divisible daughter cells. Contrastingly, monotreme zygotes, like those of birds and reptiles, undergo meroblastic (partial) division. This means the cells at the yolk's edge have cytoplasm continuous with that of the egg, allowing the yolk and embryo to exchange waste and nutrients with the surrounding cytoplasm.[15]
Physiology
Monotremes' metabolic rate is remarkably low by mammalian standards. The platypus has an average body temperature of about 31 °C (88 °F) rather than the averages of 35 °C (95 °F) for marsupials and 37 °C (99 °F) for placental mammals.[27][28] Research suggests this has been a gradual adaptation to the harsh, marginal environmental niches in which the few extant monotreme species have managed to survive, rather than a general characteristic of extinct monotremes.[29][30]
Monotremes may have less developed thermoregulation than other mammals, but recent research shows that they easily maintain a constant body temperature in a variety of circumstances, such as the platypus in icy mountain streams. Early researchers were misled by two factors: firstly, monotremes maintain a lower average temperature than most mammals; secondly, the short-beaked echidna, much easier to study than the reclusive platypus, maintains normal temperature only when active; during cold weather, it conserves energy by "switching off" its temperature regulation. Understanding of this mechanism came when reduced thermal regulation was observed in the hyraxes, which are placental mammals.
The echidna was originally thought to experience no rapid eye movement sleep.[31] However, a more recent study showed that REM sleep accounted for about 15% of sleep time observed on subjects at an environmental temperature of 25 °C (77 °F). Surveying a range of environmental temperatures, the study observed very little REM at reduced temperatures of 15 °C (59 °F) and 20 °C (68 °F), and also a substantial reduction at the elevated temperature of 28 °C (82 °F).[32]
Monotreme milk contains a highly expressed antibacterial protein not found in other mammals, perhaps to compensate for the more septic manner of milk intake associated with the absence of nipples.[33]
During the course of evolution the monotremes have lost the gastric glands normally found in mammalian stomachs as an adaptation to their diet.[34] Monotremes synthesize L-ascorbic acid only in the kidneys.[35]
Both the platypus and echidna species have spurs on their hind limbs. The echidna spurs are vestigial and have no known function, while the platypus spurs contain venom.[36] Molecular data show that the main component of platypus venom emerged before the divergence of platypus and echidnas, suggesting that the most recent common ancestor of these taxa was also possibly a venomous monotreme.[37]
Taxonomy
The traditional "theria hypothesis" states that the divergence of the monotreme lineage from the Metatheria (marsupial) and Eutheria (placental mammal) lineages happened prior to the divergence between marsupials and placental mammals, and this explains why monotremes retain a number of primitive traits presumed to have been present in the synapsid ancestors of later mammals, such as egg-laying.[38][39][40] Most morphological evidence supports the theria hypothesis, but one possible exception is a similar pattern of tooth replacement seen in monotremes and marsupials, which originally provided the basis for the competing "Marsupionta" hypothesis in which the divergence between monotremes and marsupials happened later than the divergence between these lineages and the placental mammals. Van Rheede (2005) concluded that the genetic evidence favors the theria hypothesis,[41] and this hypothesis continues to be the more widely accepted one.[42]
Monotremes are conventionally treated as comprising a single order Monotremata. The entire grouping is also traditionally placed into a subclass Prototheria, which was extended to include several fossil orders, but these are no longer seen as constituting a group allied to monotreme ancestry. A controversial hypothesis now relates the monotremes to a different assemblage of fossil mammals in a clade termed Australosphenida, a group of mammals from the Jurassic and Cretaceous of Madagascar, South America and Australia, that share tribosphenic molars.[5][43] However in a 2022 review of monotreme evolution, it was noted that Teinolophos, the oldest (Barremian ~ 125 million years ago) and the most primitive monotreme differed substantially from non-monotreme australosphenidans in having five molars as opposed to the three present in non-monotreme australosphenidians. Aptian and Cenomanian monotremes of the family Kollikodontidae (113-96.6 ma) have four molars. This suggests that the monotremes are likely to be unrelated to the australosphenidan tribosphenids.[44]
The time when the monotreme line diverged from other mammalian lines is uncertain, but one survey of genetic studies gives an estimate of about 220 million years ago,[45] while others have posited younger estimates of 163 to 186 million years ago. Teinolophos like modern monotremes displays adaptations to elongation and increased sensory perception in the jaws, related to mechanoreception or electroreception.[44]
A fossil jaw fragment attributed to a platypus from Cenomanian deposits (100-96.6 ma) from the Griman Creek Formation in Lightning Ridge, New South Wales, is the oldest platypus-like fossil.[44] The durophagous Kollikodon, the pseudotribosphenic Steropodon, and Stirtodon occur in the same Cenomanian deposits. Oligo-Miocene fossils of the toothed platypus Obdurodon have also been recovered from Australia, and fossils of a 63 million-year old platypus occur in southern Argentina (Monotrematum), see fossil monotremes below. The platypus genus Ornithorhynchus in known from Pliocene deposits, and the oldest fossil tachyglossids are Pleistocene (1.7 ma) in age.[44]
Molecular clock and fossil dating give a wide range of dates for the split between echidnas and platypuses, with one survey putting the split at 19–48 million years ago,[46] but another putting it at 17–89 million years ago.[47] It has been suggested that both the short-beaked and long-beaked echidna species are derived from a platypus-like ancestor.[44]
The precise relationships among extinct groups of mammals and modern groups such as monotremes are uncertain, but cladistic analyses usually put the last common ancestor (LCA) of placentals and monotremes close to the LCA of placentals and multituberculates, whereas some suggest that the LCA of placentals and multituberculates was more recent than the LCA of placentals and monotremes.[48][49]
- ORDER MONOTREMATA
- Family Ornithorhynchidae: platypus
- Genus Ornithorhynchus
- Platypus, O. anatinus
- Genus Ornithorhynchus
- Family Tachyglossidae: echidnas
- Genus Tachyglossus
- Short-beaked echidna, T. aculeatus
- T. a. aculeatus (Common short-beaked echidna)
- T. a. acanthion (Northern short-beaked echidna)
- T. a. lawesii (New Guinea short-beaked echidna)
- T. a. multiaculeatus (Kangaroo Island short-beaked echidna)
- T. a. setosus (Tasmanian short-beaked echidna)
- Short-beaked echidna, T. aculeatus
- Genus Zaglossus
- Sir David's long-beaked echidna, Z. attenboroughi
- Eastern long-beaked echidna, Z. bartoni
- Z. b. bartoni
- Z. b. clunius
- Z. b. diamondi
- Z. b. smeenki
- Western long-beaked echidna, Z. bruijni
- Genus Tachyglossus
- Family Ornithorhynchidae: platypus
Fossil monotremes
The first Mesozoic monotreme to be discovered was the Cenomanian (100-96.6 ma) Steropodon galmani from Lightning Ridge, New South Wales.[50] Biochemical and anatomical evidence suggests that the monotremes diverged from the mammalian lineage before the marsupials and placental mammals arose. The only Mesozoic monotremes are Teinolophos (Barremian, 126 ma), Sundrius and Kryoryctes (Albian, 113-108 ma), Steropodon, Stirtodon, Kollikodon, and an unnamed ornithorhynchid (all Cenomanian) from Australian deposits in the Cretaceous, indicating that monotremes were diversifiying by the early Late Cretaceous.[51] Monotremes have been found in the latest Cretaceous and Paleocene of southern South America, so one hypothesis is that monotremes arose in Australia in the Late Jurassic or Early Cretaceous, and that some migrated across Antarctica to South America, both of which were still united with Australia at that time.[52][53]
Fossil species
Excepting Ornithorhynchus anatinus, all the animals listed in this section are known only from fossils.
- Family Incertae sedis
- Genus Kryoryctes
- Species Kryoryctes cadburyi
- Genus Patagorhynchus
- Species Patagorhynchus pascuali - Maastrichtian, earliest known South American monotreme[53]
- Genus Kryoryctes
- Family Steropodontidae – paraphyletic assemblage
- Genus Steropodon
- Species Steropodon galmani
- Genus Teinolophos
- Species Teinolophos trusleri – 123 million years old, oldest monotreme specimen
- Genus Steropodon
- Family Ornithorhynchidae
- Genus Ornithorhynchus – oldest Ornithorhynchus specimen 9 million years old
- Species Ornithorhynchus anatinus (platypus) – oldest specimen 10,000 years old
- Genus Obdurodon – includes a number of Miocene (5–24 million years ago) Riversleigh platypuses)
- Species Obdurodon dicksoni
- Species Obdurodon insignis
- Species Obdurodon tharalkooschild – Middle Miocene and Upper Miocene (15–5 mya)
- Genus Monotrematum
- Species Monotrematum sudamericanum – 61 million years old, southern South America
- Genus Ornithorhynchus – oldest Ornithorhynchus specimen 9 million years old
- Family Tachyglossidae
- Genus Zaglossus – Upper Pleistocene (0.1–1.8 million years ago)
- Species Zaglossus robustus
- Genus Murrayglossus
- Species Murrayglossus hacketti
- Genus Megalibgwilia
- Species Megalibgwilia ramsayi – Late Pleistocene
- Species Megalibgwilia robusta – Miocene
- Genus Zaglossus – Upper Pleistocene (0.1–1.8 million years ago)
References
- Chimento, N.R.; Agnolín, F.L.; et al. (16 February 2023). "First monotreme from the Late Cretaceous of South America". Communications Biology. 6: 146. doi:10.1038/s42003-023-04498-7.
Further reading
- Nowak, Ronald M. (1999). Walker's Mammals of the World (6th ed.). Baltimore, MD: Johns Hopkins University Press. ISBN 978-0-8018-5789-8. LCCN 98023686.
External links
- "Introduction to Monotremes". U.C. Museum of Peleontology. University of California – Berkeley.
https://en.wikipedia.org/wiki/Monotreme
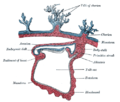
Chorion
| Chorion | |
|---|---|
 Diagram showing the chorion of a chicken egg | |
 Human fetus, enclosed in the amnion | |
| Details | |
| Identifiers | |
| Latin | chorion |
| MeSH | D002823 |
| TE | E5.11.3.1.1.0.3 |
| Anatomical terminology | |
The chorion is the outermost fetal membrane around the embryo in mammals, birds and reptiles (amniotes). It develops from an outer fold on the surface of the yolk sac, which lies outside the zona pellucida (in mammals), known as the vitelline membrane in other animals. In insects it is developed by the follicle cells while the egg is in the ovary.[1]
Structure
In humans and other mammals (excluding monotremes), the chorion is one of the fetal membranes that exist during pregnancy between the developing fetus and mother. The chorion and the amnion together form the amniotic sac. In humans it is formed by extraembryonic mesoderm and the two layers of trophoblast that surround the embryo and other membranes; the chorionic villi emerge from the chorion, invade the endometrium, and allow the transfer of nutrients from maternal blood to fetal blood.
Layers
The chorion consists of two layers: an outer formed by the trophoblast, and an inner formed by the somatic mesoderm.
The trophoblast is made up of an internal layer of cubical or prismatic cells, the cytotrophoblast or layer of Langhans, and an external multinucleated layer, the syncytiotrophoblast.
Growth
The chorion undergoes rapid proliferation and forms numerous processes, the chorionic villi, which invade and destroy the uterine decidua, while simultaneously absorbing nutritive materials from it for the growth of the embryo.
The chorionic villi are at first small and non-vascular, and consist of the trophoblast only, but they increase in size and ramify, whereas the mesoderm, carrying branches of the umbilical vessels, grows into them, and they are vascularized.
Blood is carried to the villi by the paired umbilical arteries, which branch into chorionic arteries and enter the chorionic villi as cotyledon arteries. After circulating through the capillaries of the villi, the blood is returned to the embryo by the umbilical vein. Until about the end of the second month of pregnancy, the villi cover the entire chorion, and are almost uniform in size; but, after this, they develop unequally.
Parts
The part of the chorion that is in contact with the decidua capsularis undergoes atrophy, so that by the fourth month scarcely a trace of the villi is left. This part of the chorion becomes smooth,[2] and is named the chorion laeve (from the Latin word levis, meaning smooth). As it takes no share in the formation of the placenta, this is also named the non-placental part of the chorion. As the chorion grows, the chorion laeve comes in contact with the decidua parietalis and these layers fuse.
The villi at the embryonic pole, which is in contact with the decidua basalis, increase greatly in size and complexity, and hence this part is named the chorion frondosum.[2]
Thus the placenta develops from the chorion frondosum and the decidua basalis.
Monochorionic twins
Monochorionic twins are twins that share the same placenta. This occurs in 0.3% of all pregnancies,[3] and in 75% of monozygotic (identical) twins, when the split takes place on or after the third day after fertilization.[4] The remaining 25% of monozygous twins become dichorionic diamniotic.[4] The condition may affect any type of multiple birth, resulting in monochorionic multiples.
Infections
Recent studies indicate that the chorion may be susceptible to pathogenic infections.[5] Recent findings indicate that Ureaplasma parvum bacteria can infect the chorion tissue, thereby impacting pregnancy outcome.[6] In addition, footprints of JC polyomavirus and Merkel cell polyomavirus have been detected in chorionic villi from females affected by spontaneous abortion as well as pregnant women.[7][8] Another virus, BK polyomavirus has been detected in the same tissues, but with lesser extent.[9]
Other animals
In reptiles, birds, and monotremes, the chorion is one of the four extraembryonic membranes that make up the amniotic egg that provide for the nutrients and protection needed for the embryo's survival. It is located inside the albumen, which is the white of the egg. It encloses the embryo and the rest of the embryonic system. The chorion is also present in insects. During growth and development of the embryo, there is an increased need for oxygen. To compensate for this, the chorion and the allantois fuse together to form the chorioallantoic membrane. Together these form a double membrane, which functions to remove carbon dioxide and to replenish oxygen through the porous shell. At the time of hatching, the fetus becomes detached from the chorion as it emerges from the shell.
Additional images
Diagram illustrating early formation of allantois and differentiation of body-stalk.
See also
- Choriogenesis
- Chorioamnionitis, an inflammation of the chorion and amnion, usually due to bacterial infection
- Chorionic hematoma
- Gestational trophoblastic disease, any abnormal proliferation of the trophoblasts, including choriocarcinoma, a highly invasive cancer.
References
![]() This article incorporates text in the public domain from page 60 of the 20th edition of Gray's Anatomy (1918)
This article incorporates text in the public domain from page 60 of the 20th edition of Gray's Anatomy (1918)
- Tagliapietra A, Rotondo JC, Bononi I, Mazzoni E, Magagnoli F, Maritati M (2019). "Footprints of BK and JC polyomaviruses in specimens from females affected by spontaneous abortion". Hum Reprod. 34 (3): 433–440. doi:10.1002/jcp.27490. hdl:11392/2397717. PMID 30590693. S2CID 53106591.
External links
- Histology image: 19903loa – Histology Learning System at Boston University — "Female Reproductive System: placenta, chorionic plate"
- McGill
https://en.wikipedia.org/wiki/Chorion
Geomagnetic reversal
A geomagnetic reversal is a change in a planet's magnetic field such that the positions of magnetic north and magnetic south are interchanged (not to be confused with geographic north and geographic south). The Earth's field has alternated between periods of normal polarity, in which the predominant direction of the field was the same as the present direction, and reverse polarity, in which it was the opposite. These periods are called chrons.
Reversal occurrences are statistically random. There have been at least 183 reversals over the last 83 million years (on average once every ~450,000 years). The latest, the Brunhes–Matuyama reversal, occurred 780,000 years ago,[1] with widely varying estimates of how quickly it happened. Other sources estimate that the time that it takes for a reversal to complete is on average around 7,000 years for the four most recent reversals.[2] Clement (2004) suggests that this duration is dependent on latitude, with shorter durations at low latitudes, and longer durations at mid and high latitudes.[2] Although variable, the duration of a full reversal is typically between 2,000 and 12,000 years.[3]
Although there have been periods in which the field reversed globally (such as the Laschamp excursion) for several hundred years,[4] these events are classified as excursions rather than full geomagnetic reversals. Stable polarity chrons often show large, rapid directional excursions, which occur more often than reversals, and could be seen as failed reversals. During such an excursion, the field reverses in the liquid outer core, but not in the solid inner core. Diffusion in the liquid outer core is on timescales of 500 years or less, while that of the solid inner core is longer, around 3,000 years.[5]
History
In the early 20th century, geologists such as Bernard Brunhes first noticed that some volcanic rocks were magnetized opposite to the direction of the local Earth's field. The first systematic evidence for and time-scale estimate of the magnetic reversals were made by Motonori Matuyama in the late 1920s; he observed that rocks with reversed fields were all of early Pleistocene age or older. At the time, the Earth's polarity was poorly understood, and the possibility of reversal aroused little interest.[6][7]
Three decades later, when Earth's magnetic field was better understood, theories were advanced suggesting that the Earth's field might have reversed in the remote past. Most paleomagnetic research in the late 1950s included an examination of the wandering of the poles and continental drift. Although it was discovered that some rocks would reverse their magnetic field while cooling, it became apparent that most magnetized volcanic rocks preserved traces of the Earth's magnetic field at the time the rocks had cooled. In the absence of reliable methods for obtaining absolute ages for rocks, it was thought that reversals occurred approximately every million years.[6][7]
The next major advance in understanding reversals came when techniques for radiometric dating were improved in the 1950s. Allan Cox and Richard Doell, at the United States Geological Survey, wanted to know whether reversals occurred at regular intervals, and invited the geochronologist Brent Dalrymple to join their group. They produced the first magnetic-polarity time scale in 1959. As they accumulated data, they continued to refine this scale in competition with Don Tarling and Ian McDougall at the Australian National University. A group led by Neil Opdyke at the Lamont–Doherty Earth Observatory showed that the same pattern of reversals was recorded in sediments from deep-sea cores.[7]
During the 1950s and 1960s information about variations in the Earth's magnetic field was gathered largely by means of research vessels, but the complex routes of ocean cruises rendered the association of navigational data with magnetometer readings difficult. Only when data were plotted on a map did it become apparent that remarkably regular and continuous magnetic stripes appeared on the ocean floors.[6][7]
In 1963, Frederick Vine and Drummond Matthews provided a simple explanation by combining the seafloor spreading theory of Harry Hess with the known time scale of reversals: new sea floor is magnetized in the direction of the then-current field. Thus, sea floor spreading from a central ridge will produce pairs of magnetic stripes parallel to the ridge.[8] Canadian L. W. Morley independently proposed a similar explanation in January 1963, but his work was rejected by the scientific journals Nature and Journal of Geophysical Research, and remained unpublished until 1967, when it appeared in the literary magazine Saturday Review.[6] The Morley–Vine–Matthews hypothesis was the first key scientific test of the seafloor spreading theory of continental drift.[7]
Beginning in 1966, Lamont–Doherty Geological Observatory scientists found that the magnetic profiles across the Pacific-Antarctic Ridge were symmetrical and matched the pattern in the north Atlantic's Reykjanes ridge. The same magnetic anomalies were found over most of the world's oceans, which permitted estimates for when most of the oceanic crust had developed.[6][7]
Observing past fields
Past field reversals are recorded in the "frozen" ferromagnetic (more accurately ferrimagnetic) minerals of consolidated sedimentary deposits or cooled volcanic flows on land.
The past record of geomagnetic reversals was first noticed by observing the magnetic stripe "anomalies" on the ocean floor. Lawrence W. Morley, Frederick John Vine and Drummond Hoyle Matthews made the connection to seafloor spreading in the Morley–Vine–Matthews hypothesis[8][9] which soon led to the development of the theory of plate tectonics. The relatively constant rate at which the sea floor spreads results in substrate "stripes" from which past magnetic field polarity can be inferred from a magnetometer towed along the sea floor.
Because no existing unsubducted sea floor (or sea floor thrust onto continental plates) is more than about 180 million years (Ma) old, other methods are necessary for detecting older reversals. Most sedimentary rocks incorporate tiny amounts of iron rich minerals, whose orientation is influenced by the ambient magnetic field at the time at which they formed. These rocks can preserve a record of the field if it is not later erased by chemical, physical or biological change.
Because Earth's magnetic field is a global phenomenon, similar patterns of magnetic variations at different sites may be used to help calculate age in different locations. The past four decades of paleomagnetic data about seafloor ages (up to ~250 Ma) has been useful in estimating the age of geologic sections elsewhere. While not an independent dating method, it depends on "absolute" age dating methods like radioisotopic systems to derive numeric ages. It has become especially useful to metamorphic and igneous geologists where index fossils are seldom available.
Geomagnetic polarity time scale
Through analysis of seafloor magnetic anomalies and dating of reversal sequences on land, paleomagnetists have been developing a Geomagnetic Polarity Time Scale (GPTS). The current time scale contains 184 polarity intervals in the last 83 million years (and therefore 183 reversals).[10][11]
Changing frequency over time
The rate of reversals in the Earth's magnetic field has varied widely over time. 72 million years ago (Ma), the field reversed 5 times in a million years. In a 4-million-year period centered on 54 Ma, there were 10 reversals; at around 42 Ma, 17 reversals took place in the span of 3 million years. In a period of 3 million years centering on 24 Ma, 13 reversals occurred. No fewer than 51 reversals occurred in a 12-million-year period, centering on 15 million years ago. Two reversals occurred during a span of 50,000 years. These eras of frequent reversals have been counterbalanced by a few "superchrons" – long periods when no reversals took place.[12]
Superchrons
A superchron is a polarity interval lasting at least 10 million years. There are two well-established superchrons, the Cretaceous Normal and the Kiaman. A third candidate, the Moyero, is more controversial. The Jurassic Quiet Zone in ocean magnetic anomalies was once thought to represent a superchron, but is now attributed to other causes.
The Cretaceous Normal (also called the Cretaceous Superchron or C34) lasted for almost 40 million years, from about 120 to 83 million years ago, including stages of the Cretaceous period from the Aptian through the Santonian. The frequency of magnetic reversals steadily decreased prior to the period, reaching its low point (no reversals) during the period. Between the Cretaceous Normal and the present, the frequency has generally increased slowly.[13]
The Kiaman Reverse Superchron lasted from approximately the late Carboniferous to the late Permian, or for more than 50 million years, from around 312 to 262 million years ago.[13] The magnetic field had reversed polarity. The name "Kiaman" derives from the Australian town of Kiama, where some of the first geological evidence of the superchron was found in 1925.[14]
The Ordovician is suspected to have hosted another superchron, called the Moyero Reverse Superchron, lasting more than 20 million years (485 to 463 million years ago). Thus far, this possible superchron has only been found in the Moyero river section north of the polar circle in Siberia.[15] Moreover, the best data from elsewhere in the world do not show evidence for this superchron.[16]
Certain regions of ocean floor, older than 160 Ma, have low-amplitude magnetic anomalies that are hard to interpret. They are found off the east coast of North America, the northwest coast of Africa, and the western Pacific. They were once thought to represent a superchron called the Jurassic Quiet Zone, but magnetic anomalies are found on land during this period. The geomagnetic field is known to have low intensity between about 130 Ma and 170 Ma, and these sections of ocean floor are especially deep, causing the geomagnetic signal to be attenuated between the seabed and the surface.[16]
Statistical properties of reversals
Several studies have analyzed the statistical properties of reversals in the hope of learning something about their underlying mechanism. The discriminating power of statistical tests is limited by the small number of polarity intervals. Nevertheless, some general features are well established. In particular, the pattern of reversals is random. There is no correlation between the lengths of polarity intervals.[17] There is no preference for either normal or reversed polarity, and no statistical difference between the distributions of these polarities. This lack of bias is also a robust prediction of dynamo theory.[13]
There is no rate of reversals, as they are statistically random. The randomness of the reversals is inconsistent with periodicity, but several authors have claimed to find periodicity.[18] However, these results are probably artifacts of an analysis using sliding windows to attempt to determine reversal rates.[19]
Most statistical models of reversals have analyzed them in terms of a Poisson process or other kinds of renewal process. A Poisson process would have, on average, a constant reversal rate, so it is common to use a non-stationary Poisson process. However, compared to a Poisson process, there is a reduced probability of reversal for tens of thousands of years after a reversal. This could be due to an inhibition in the underlying mechanism, or it could just mean that some shorter polarity intervals have been missed.[13] A random reversal pattern with inhibition can be represented by a gamma process. In 2006, a team of physicists at the University of Calabria found that the reversals also conform to a Lévy distribution, which describes stochastic processes with long-ranging correlations between events in time.[20][21] The data are also consistent with a deterministic, but chaotic, process.[22]
Character of transitions
Duration
Most estimates for the duration of a polarity transition are between 1,000 and 10,000 years,[13] but some estimates are as quick as a human lifetime.[23] Studies of 16.7-million-year-old lava flows on Steens Mountain, Oregon, indicate that the Earth's magnetic field is capable of shifting at a rate of up to 6 degrees per day.[24] This was initially met with skepticism from paleomagnetists. Even if changes occur that quickly in the core, the mantle, which is a semiconductor, is thought to remove variations with periods less than a few months. A variety of possible rock magnetic mechanisms were proposed that would lead to a false signal.[25] However, paleomagnetic studies of other sections from the same region (the Oregon Plateau flood basalts) give consistent results.[26][27] It appears that the reversed-to-normal polarity transition that marks the end of Chron C5Cr (16.7 million years ago) contains a series of reversals and excursions.[28] In addition, geologists Scott Bogue of Occidental College and Jonathan Glen of the US Geological Survey, sampling lava flows in Battle Mountain, Nevada, found evidence for a brief, several-year-long interval during a reversal when the field direction changed by over 50 degrees. The reversal was dated to approximately 15 million years ago.[29][30] In August 2018, researchers reported a reversal lasting only 200 years.[31] But a 2019 paper estimated that the most recent reversal, 780,000 years ago, lasted 22,000 years.[32][33]
Magnetic field
The magnetic field will not vanish completely, but many poles might form chaotically in different places during reversal, until it stabilizes again.[34][35]
Causes
The magnetic field of the Earth, and of other planets that have magnetic fields, is generated by dynamo action in which convection of molten iron in the planetary core generates electric currents which in turn give rise to magnetic fields.[13] In simulations of planetary dynamos, reversals often emerge spontaneously from the underlying dynamics. For example, Gary Glatzmaier and collaborator Paul Roberts of UCLA ran a numerical model of the coupling between electromagnetism and fluid dynamics in the Earth's interior. Their simulation reproduced key features of the magnetic field over more than 40,000 years of simulated time and the computer-generated field reversed itself.[36][37] Global field reversals at irregular intervals have also been observed in the laboratory liquid metal experiment "VKS2".[38]
In some simulations, this leads to an instability in which the magnetic field spontaneously flips over into the opposite orientation. This scenario is supported by observations of the solar magnetic field, which undergoes spontaneous reversals every 9–12 years. However, with the Sun it is observed that the solar magnetic intensity greatly increases during a reversal, whereas reversals on Earth seem to occur during periods of low field strength.[39]
Hypothesized triggers
Some scientists, such as Richard A. Muller, think that geomagnetic reversals are not spontaneous processes but rather are triggered by external events that directly disrupt the flow in the Earth's core. Proposals include impact events[40][41] or internal events such as the arrival of continental slabs carried down into the mantle by the action of plate tectonics at subduction zones or the initiation of new mantle plumes from the core-mantle boundary.[42] Supporters of this hypothesis hold that any of these events could lead to a large scale disruption of the dynamo, effectively turning off the geomagnetic field. Because the magnetic field is stable in either the present north–south orientation or a reversed orientation, they propose that when the field recovers from such a disruption it spontaneously chooses one state or the other, such that half the recoveries become reversals. However, the proposed mechanism does not appear to work in a quantitative model, and the evidence from stratigraphy for a correlation between reversals and impact events is weak. There is no evidence for a reversal connected with the impact event that caused the Cretaceous–Paleogene extinction event.[43]
Effects on biosphere
Shortly after the first geomagnetic polarity time scales were produced, scientists began exploring the possibility that reversals could be linked to extinctions. Most such proposals rest on the assumption that the Earth's magnetic field would be much weaker during reversals. Possibly the first such hypothesis was that high-energy particles trapped in the Van Allen radiation belt could be liberated and bombard the Earth.[44][45] Detailed calculations confirm that if the Earth's dipole field disappeared entirely (leaving the quadrupole and higher components), most of the atmosphere would become accessible to high-energy particles, but would act as a barrier to them, and cosmic ray collisions would produce secondary radiation of beryllium-10 or chlorine-36. A 2012 German study of Greenland ice cores showed a peak of beryllium-10 during a brief complete reversal 41,000 years ago, which led to the magnetic field strength dropping to an estimated 5% of normal during the reversal.[46] There is evidence that this occurs both during secular variation[47][48] and during reversals.[49][50]
Another hypothesis by McCormac and Evans assumes that the Earth's field disappears entirely during reversals.[51] They argue that the atmosphere of Mars may have been eroded away by the solar wind because it had no magnetic field to protect it. They predict that ions would be stripped away from Earth's atmosphere above 100 km. However, paleointensity measurements show that the magnetic field has not disappeared during reversals. Based on paleointensity data for the last 800,000 years,[52] the magnetopause is still estimated to have been at about three Earth radii during the Brunhes-Matuyama reversal.[44] Even if the internal magnetic field did disappear, the solar wind can induce a magnetic field in the Earth's ionosphere sufficient to shield the surface from energetic particles.[53]
Hypotheses have also advanced toward linking reversals to mass extinctions.[54] Many such arguments were based on an apparent periodicity in the rate of reversals, but more careful analyses show that the reversal record is not periodic.[19] It may be, however, that the ends of superchrons have caused vigorous convection leading to widespread volcanism, and that the subsequent airborne ash caused extinctions.[55]
Tests of correlations between extinctions and reversals are difficult for a number of reasons. Larger animals are too scarce in the fossil record for good statistics, so paleontologists have analyzed microfossil extinctions. Even microfossil data can be unreliable if there are hiatuses in the fossil record. It can appear that the extinction occurs at the end of a polarity interval when the rest of that polarity interval was simply eroded away.[25] Statistical analysis shows no evidence for a correlation between reversals and extinctions.[56][44]
See also
- List of geomagnetic reversals, including ages
- Magnetic anomaly
References
- Plotnick, Roy E. (1 January 1980). "Relationship between biological extinctions and geomagnetic reversals". Geology. 8 (12): 578. Bibcode:1980Geo.....8..578P. doi:10.1130/0091-7613(1980)8<578:RBBEAG>2.0.CO;2.
Further reading
- Barry, Patrick (11 May 2006). "Ships' logs give clues to Earth's magnetic decline". New Scientist. Retrieved 8 January 2019.
- Hoffman, Kenneth A. (18 July 1995). "How Are Geomagnetic Reversals Related to Field Intensity?". EOS. 76: 289. doi:10.1029/95EO00172. Archived from the original on 16 March 2009.
- Jacobs, J. A. (1994). Reversals of the Earth's magnetic field (2nd ed.). Cambridge University Press. ISBN 978-0521450720.
- Ogg, J. G. (2012). "Geomagnetic polarity time scale". In Gradstein, F. M.; Ogg, J. G.; Schmitz, Mark; Ogg, Gabi (eds.). The geologic time scale 2012. Volume 2 (1st ed.). Elsevier. pp. 85–114. ISBN 978-0444594259.
- Okada, Makoto; Niitsuma, Nobuaki (July 1989). "Detailed paleomagnetic records during the Brunhes-Matuyama geomagnetic reversal, and a direct determination of depth lag for magnetization in marine sediments". Physics of the Earth and Planetary Interiors. 56 (1–2): 133–150. Bibcode:1989PEPI...56..133O. doi:10.1016/0031-9201(89)90043-5.
- Opdyke, Neil D. (1996). Magnetic stratigraphy. Academic Press. ISBN 978-0080535722.
- "Look down, look up, look out!". The Economist. 10 May 2007. Retrieved 8 January 2019.
- Turner, Gillian (2011). North Pole, South Pole: The epic quest to solve the great mystery of Earth's magnetism. New York, NY: The Experiment. ISBN 9781615190317.
External links
- Is it true that the Earth's magnetic field is about to flip? physics.org, accessed 8 January 2019
- Pole Reversal Happens All The (Geologic) Time NASA, accessed 1 March 2022
https://en.wikipedia.org/wiki/Geomagnetic_reversal

















No comments:
Post a Comment