| Saara hardwickii | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Reptilia |
| Order: | Squamata |
| Suborder: | Iguania |
| Family: | Agamidae |
| Genus: | Saara |
| Species: | S. hardwickii
|
| Binomial name | |
| Saara hardwickii (Gray, 1827)
| |
| Synonyms[2][3] | |
Saara hardwickii, commonly known as Hardwicke's spiny-tailed lizard or the Indian spiny-tailed lizard is a species of lizard in the family Agamidae. The species is found in patches across the Thar desert, Kutch, and surrounding arid zones in India and Pakistan. It is mainly herbivorous and lives in numbers in some areas. Since it is found in loose clusters it often attracts predators such as raptors. It is also hunted by local peoples in the belief that the fat extracted from it is an aphrodisiac.
https://en.wikipedia.org/wiki/Saara_hardwickii
| 3rd Colorado Cavalry Regiment | |
|---|---|
 The unit at the Sand Creek massacre | |
| Active | August 20, 1864 – December 31, 1864 |
| Disbanded | December 31, 1864 |
| Country | |
| Allegiance | |
| Branch | Cavalry |
| Garrison/HQ | Denver, Colorado |
| Nickname(s) | Bloodless Third |
| Engagements | American Civil War |
| Commanders | |
| Notable commanders | Colonel George L. Shoup Colonel John M. Chivington (as District commander) |
The 3rd Colorado Cavalry Regiment was a Union Army unit formed in the mid-1860s when increased traffic on the United States emigrant trails and settler encroachment resulted in numerous attacks against them by the Cheyenne and Arapaho. The Hungate massacre and the display in Denver of mutilated victims raised political pressure for the government to protect its people. Governor John Evans sought and gained authorization from the War Department in Washington to found the Third. More a militia than a military unit, the "Bloodless Third" was composed of "100-daysers," that is, volunteers who signed on for 100 days to fight against the Indians. (Its nickname came from its lack of battle experience.) The unit's only commander was Col. George L. Shoup, a politician from Colorado.[1][2] The regiment was assigned to the District of Colorado commanded by Col. John M. Chivington.
https://en.wikipedia.org/wiki/3rd_Colorado_Cavalry_Regiment
List of reptiles of Mongolia
- Kaspischer even-fingered gecko or squeaky pygmy gecko (Alsophylax pipiens)
- Yangihissar gecko (Tenuidactylus elongatus)
- Przewalski's wonder gecko or plate-tailed gecko (Teratoscincus przewalskii)
Family Agamidae
- Mongolian agama or Mongolian rock agama (Paralaudakia stoliczkana)
- Sunwatcher toad-head agama (Phrynocephalus helioscopus)
- Tuva toad-head agama (Phrynocephalus versicolor)
Family Lacertidae
- Mongolian racerunner (Eremias argus)
- Stepperunner or arguta (Eremias arguta)
- Dzungarian racerunner (Eremias dzungarica)
- Multi-oscillated racerunner (Eremias multiocellata)
- Gobi racerunner (Eremias przewalskii)
- Variegated racerunner (Eremias vermiculata)
- Sand lizard (Lacerta agilis)
- Viviparous lizard or common lizard (Zootoca vivipara)
Family Boidae
- Tatary sand boa (Eryx tataricus)
Family Colubridae
- Elaphe schrenckii
- European grass snake or grass snake (Natrix natrix)
- Steppe ribbon racer (Psammophis lineolatus)
Family Viperidae
- Halys pit viper or Asian viper (Gloydius halys)
- Ussuri mamushi (Gloydius ussuriensis)
- Adder or common northern viper (Vipera berus)
Sources
https://en.wikipedia.org/wiki/List_of_reptiles_of_Mongolia
Mortar is a workable paste which hardens to bind building blocks such as stones, bricks, and concrete masonry units, to fill and seal the irregular gaps between them, spread the weight of them evenly, and sometimes to add decorative colors or patterns to masonry walls. In its broadest sense, mortar includes pitch, asphalt, and soft mud or clay, as those used between mud bricks, as well as cement mortar. The word "mortar" comes from Old French mortier, "builder's mortar, plaster; bowl for mixing." (13c.).[1]
Cement mortar becomes hard when it cures, resulting in a rigid aggregate structure; however, the mortar functions as a weaker component than the building blocks and serves as the sacrificial element in the masonry, because mortar is easier and less expensive to repair than the building blocks. Bricklayers typically make mortars using a mixture of sand, a binder, and water. The most common binder since the early 20th century is Portland cement, but the ancient binder lime mortar is still used in some specialty new construction. Lime, lime mortar, and gypsum in the form of plaster of Paris are used particularly in the repair and repointing of historic buildings and structures, so that the repair materials will be similar in performance and appearance to the original materials. Several types of cement mortars and additives exist.
https://en.wikipedia.org/wiki/Mortar_(masonry)
The Benghazi burner or Benghazi cooker was an improvised petrol stove or brazier used by British Army troops and their Commonwealth and Imperial allies in the Second World War, during and after the North African Campaign.
https://en.wikipedia.org/wiki/Benghazi_burner
| Psychodidae Temporal range:
| |
|---|---|

| |
| Male Clogmia albipunctata. A moth-like dense coat of small hairs gives rise to the term "moth fly". | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Diptera |
| Suborder: | Nematocera |
| Infraorder: | Psychodomorpha |
| Superfamily: | Psychodoidea |
| Family: | Psychodidae Newman, 1834[1] |
| Synonyms | |
|
Phlebotomidae | |
Psychodidae, also called drain flies, sink flies, filter flies,[2] sewer flies, or sewer gnats, is a family of true flies. Some genera have short, hairy bodies and wings, giving them a "furry" moth-like appearance, hence one of their common names, moth flies.[2] Members of the sub-family Phlebotominae, which are hematophagous (feed on blood), may be called sand flies in some countries, although this term is also used for other unrelated flies.
There are more than 2,600 described species worldwide, most of them native to the humid tropics. This makes them one of the most diverse families of their order.[3] Drain flies sometimes inhabit plumbing drains and sewage systems, where they are harmless, but may be a persistent annoyance.[4]
https://en.wikipedia.org/wiki/Psychodidae
| White spruce | |
|---|---|

| |
| Mature white spruce in Fairbanks, Alaska | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Gymnosperms |
| Division: | Pinophyta |
| Class: | Pinopsida |
| Order: | Pinales |
| Family: | Pinaceae |
| Genus: | Picea |
| Species: | P. glauca
|
| Binomial name | |
| Picea glauca (Moench) Voss
| |

| |
| Natural range | |
| Synonyms[2] | |
|
| |
List |
| NCBI genome ID | 3330 |
|---|---|
| Ploidy | 2 |
| Genome size | 20 Gbp |
| Number of chromosomes | 12 |
| Year of completion | 2015 |
| Sequenced organelle | plastid and mitochondrion |
| Organelle size | 123 kbp and 5.93 Mbp |
| Year of completion | 2015 |
Picea glauca, the white spruce,[3] is a species of spruce native to the northern temperate and boreal forests in North America. Picea glauca is native from central Alaska all through the east, across western and southern/central Canada to the Avalon Peninsula in Newfoundland, and south to Montana, North Dakota, Minnesota, Wisconsin, Michigan, Upstate New York and Vermont, along with the mountainous and immediate coastal portions of New Hampshire and Maine, where temperatures are just barely cool and moist enough to support it. There is also an isolated population in the Black Hills of South Dakota and Wyoming.[4][5][1][6] It is also known as Canadian spruce, skunk spruce, cat spruce, Black Hills spruce, western white spruce, Alberta white spruce, and Porsild spruce.[7]
https://en.wikipedia.org/wiki/Picea_glauca
| Cebrennus rechenbergi | |
|---|---|

| |
| Cebrennus rechenbergi in action | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Subphylum: | Chelicerata |
| Class: | Arachnida |
| Order: | Araneae |
| Infraorder: | Araneomorphae |
| Family: | Sparassidae |
| Genus: | Cebrennus |
| Species: | C. rechenbergi
|
| Binomial name | |
| Cebrennus rechenbergi Peter Jäger, 2014
| |

| |
| Map of Morocco with the Errachidia Province in dark red, the region where Cebrennus rechenbergi lives | |
Cebrennus rechenbergi, also known as the Moroccan flic-flac spider and cartwheeling spider,[1] is a species of huntsman spider indigenous to the sand dunes of the Erg Chebbi desert in Morocco. If provoked or threatened it can escape by doubling its normal walking speed using forward or backward flips similar to acrobatic flic-flac movements used by gymnasts.[2][3] C. rechenbergi is the only spider known to use this unique form of rolling locomotion.[4] The discovery of the Moroccan flic-flac spider has influenced biomimetic robot research, resulting in the development of an experimental robot based on the spider's motion.[5][6]
https://en.wikipedia.org/wiki/Cebrennus_rechenbergi
| Quartz | |
|---|---|
 Quartz crystal cluster from Tibet | |
| General | |
| Category | silicate mineral[1] |
| Formula (repeating unit) | SiO2 |
| IMA symbol | Qz[2] |
| Strunz classification | 4.DA.05 (oxides) |
| Dana classification | 75.01.03.01 (tectosilicates) |
| Crystal system | α-quartz: trigonal β-quartz: hexagonal |
| Crystal class | α-quartz: trapezohedral (class 3 2) β-quartz: trapezohedral (class 6 2 2)[3] |
| Space group | α-quartz: P3221 (no. 154)[4] β-quartz: P6222 (no. 180) or P6422 (no. 181)[5] |
| Unit cell | a = 4.9133 Å, c = 5.4053 Å; Z=3 |
| Identification | |
| Formula mass | 60.083 g·mol−1 |
| Color | Colorless through various colors (pink, orange, purple, dark brown) to black |
| Crystal habit | 6-sided prism ending in 6-sided pyramid (typical), drusy, fine-grained to microcrystalline, massive |
| Twinning | Common Dauphine law, Brazil law and Japan law |
| Cleavage | {0110} Indistinct |
| Fracture | Conchoidal |
| Tenacity | Brittle |
| Mohs scale hardness | 7 – lower in impure varieties (defining mineral) |
| Luster | Vitreous – waxy to dull when massive |
| Streak | White |
| Diaphaneity | Transparent to nearly opaque |
| Specific gravity | 2.65; variable 2.59–2.63 in impure varieties |
| Optical properties | Uniaxial (+) |
| Refractive index | nω = 1.543–1.545 nε = 1.552–1.554 |
| Birefringence | +0.009 (B-G interval) |
| Pleochroism | None |
| Melting point | 1670 °C (β tridymite) 1713 °C (β cristobalite)[3] |
| Solubility | Insoluble at STP; 1 ppmmass at 400 °C and 500 lb/in2 to 2600 ppmmass at 500 °C and 1500 lb/in2[3] |
| Other characteristics | lattice: hexagonal, Piezoelectric, may be triboluminescent, chiral (hence optically active if not racemic) |
| References | [6][7][8][9] |
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon–oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical formula of SiO2. Quartz is the second most abundant mineral in Earth's continental crust, behind feldspar.[10]
Quartz exists in two forms, the normal α-quartz and the high-temperature β-quartz, both of which are chiral. The transformation from α-quartz to β-quartz takes place abruptly at 573 °C (846 K; 1,063 °F). Since the transformation is accompanied by a significant change in volume, it can easily induce microfracturing of ceramics or rocks passing through this temperature threshold.
There are many different varieties of quartz, several of which are classified as gemstones. Since antiquity, varieties of quartz have been the most commonly used minerals in the making of jewelry and hardstone carvings, especially in Eurasia.
Quartz is the mineral defining the value of 7 on the Mohs scale of hardness, a qualitative scratch method for determining the hardness of a material to abrasion.
https://en.wikipedia.org/wiki/Quartz
| String theory |
|---|
 |
| Fundamental objects |
| Perturbative theory |
| Non-perturbative results |
| Phenomenology |
| Mathematics |
|
|
|
|
The holographic principle is an axiom in string theories and a supposed property of quantum gravity that states that the description of a volume of space can be thought of as encoded on a lower-dimensional boundary to the region — such as a light-like boundary like a gravitational horizon.[1][2] First proposed by Gerard 't Hooft, it was given a precise string-theory interpretation by Leonard Susskind,[3] who combined his ideas with previous ones of 't Hooft and Charles Thorn.[3][4] Leonard Susskind said, “The three-dimensional world of ordinary experience––the universe filled with galaxies, stars, planets, houses, boulders, and people––is a hologram, an image of reality coded on a distant two-dimensional surface."[5] As pointed out by Raphael Bousso,[6] Thorn observed in 1978 that string theory admits a lower-dimensional description in which gravity emerges from it in what would now be called a holographic way. The prime example of holography is the AdS/CFT correspondence.
The holographic principle was inspired by black hole thermodynamics, which conjectures that the maximum entropy in any region scales with the radius squared, and not cubed as might be expected. In the case of a black hole, the insight was that the information content of all the objects that have fallen into the hole might be entirely contained in surface fluctuations of the event horizon. The holographic principle resolves the black hole information paradox within the framework of string theory.[5] However, there exist classical solutions to the Einstein equations that allow values of the entropy larger than those allowed by an area law (radius squared), hence in principle larger than those of a black hole. These are the so-called "Wheeler's bags of gold". The existence of such solutions conflicts with the holographic interpretation, and their effects in a quantum theory of gravity including the holographic principle are not yet fully understood.[7]
https://en.wikipedia.org/wiki/Holographic_principle
| String theory |
|---|
 |
| Fundamental objects |
| Perturbative theory |
| Non-perturbative results |
| Phenomenology |
| Mathematics |
|
|
|
|
In theoretical physics, the anti-de Sitter/conformal field theory correspondence, sometimes called Maldacena duality or gauge/gravity duality, is a conjectured relationship between two kinds of physical theories. On one side are anti-de Sitter spaces (AdS) which are used in theories of quantum gravity, formulated in terms of string theory or M-theory. On the other side of the correspondence are conformal field theories (CFT) which are quantum field theories, including theories similar to the Yang–Mills theories that describe elementary particles.
The duality represents a major advance in the understanding of string theory and quantum gravity.[1] This is because it provides a non-perturbative formulation of string theory with certain boundary conditions and because it is the most successful realization of the holographic principle, an idea in quantum gravity originally proposed by Gerard 't Hooft and promoted by Leonard Susskind.
It also provides a powerful toolkit for studying strongly coupled quantum field theories.[2] Much of the usefulness of the duality results from the fact that it is a strong–weak duality: when the fields of the quantum field theory are strongly interacting, the ones in the gravitational theory are weakly interacting and thus more mathematically tractable. This fact has been used to study many aspects of nuclear and condensed matter physics by translating problems in those subjects into more mathematically tractable problems in string theory.
The AdS/CFT correspondence was first proposed by Juan Maldacena in late 1997.[3] Important aspects of the correspondence were soon elaborated on in two articles, one by Steven Gubser, Igor Klebanov and Alexander Polyakov, and another by Edward Witten. By 2015, Maldacena's article had over 10,000 citations, becoming the most highly cited article in the field of high energy physics,[4] reaching over 20,000 citations in 2020.
https://en.wikipedia.org/wiki/AdS/CFT_correspondence
In mathematics, in the field of differential equations, a boundary value problem is a differential equation together with a set of additional constraints, called the boundary conditions.[1] A solution to a boundary value problem is a solution to the differential equation which also satisfies the boundary conditions.
Boundary value problems arise in several branches of physics as any physical differential equation will have them. Problems involving the wave equation, such as the determination of normal modes, are often stated as boundary value problems. A large class of important boundary value problems are the Sturm–Liouville problems. The analysis of these problems involves the eigenfunctions of a differential operator.
To be useful in applications, a boundary value problem should be well posed. This means that given the input to the problem there exists a unique solution, which depends continuously on the input. Much theoretical work in the field of partial differential equations is devoted to proving that boundary value problems arising from scientific and engineering applications are in fact well-posed.
Among the earliest boundary value problems to be studied is the Dirichlet problem, of finding the harmonic functions (solutions to Laplace's equation); the solution was given by the Dirichlet's principle.
https://en.wikipedia.org/wiki/Boundary_value_problem
A normal mode of a dynamical system is a pattern of motion in which all parts of the system move sinusoidally with the same frequency and with a fixed phase relation. The free motion described by the normal modes takes place at fixed frequencies. These fixed frequencies of the normal modes of a system are known as its natural frequencies or resonant frequencies. A physical object, such as a building, bridge, or molecule, has a set of normal modes and their natural frequencies that depend on its structure, materials and boundary conditions.
The most general motion of a system is a superposition of its normal modes. The modes are normal in the sense that they can move independently, that is to say that an excitation of one mode will never cause motion of a different mode. In mathematical terms, normal modes are orthogonal to each other.
In multivariable calculus, an initial value problem[a] (IVP) is an ordinary differential equation together with an initial condition which specifies the value of the unknown function at a given point in the domain. Modeling a system in physics or other sciences frequently amounts to solving an initial value problem. In that context, the differential initial value is an equation which specifies how the system evolves with time given the initial conditions of the problem.
https://en.wikipedia.org/wiki/Initial_value_problem
In theoretical physics, compactification means changing a theory with respect to one of its space-time dimensions. Instead of having a theory with this dimension being infinite, one changes the theory so that this dimension has a finite length, and may also be periodic.
Compactification plays an important part in thermal field theory where one compactifies time, in string theory where one compactifies the extra dimensions of the theory, and in two- or one-dimensional solid state physics, where one considers a system which is limited in one of the three usual spatial dimensions.
At the limit where the size of the compact dimension goes to zero, no fields depend on this extra dimension, and the theory is dimensionally reduced.

https://en.wikipedia.org/wiki/Compactification_(physics)
In physics, quasiparticles and collective excitations are closely related phenomena arising when a microscopically complicated system such as a solid behaves as if it contained different weakly interacting particles in vacuum.
For example, as an electron travels through a semiconductor, its motion is disturbed in a complex way by its interactions with other electrons and with atomic nuclei. The electron behaves as though it has a different effective mass travelling unperturbed in vacuum. Such an electron is called an electron quasiparticle.[1] In another example, the aggregate motion of electrons in the valence band of a semiconductor or a hole band in a metal[2] behave as though the material instead contained positively charged quasiparticles called electron holes. Other quasiparticles or collective excitations include the phonon, a quasiparticle derived from the vibrations of atoms in a solid, and the plasmons, a particle derived from plasma oscillation.
These phenomena are typically called quasiparticles if they are related to fermions, and called collective excitations if they are related to bosons,[1] although the precise distinction is not universally agreed upon.[3] Thus, electrons and electron holes (fermions) are typically called quasiparticles, while phonons and plasmons (bosons) are typically called collective excitations.
The quasiparticle concept is important in condensed matter physics because it can simplify the many-body problem in quantum mechanics. The theory of quasiparticles was started by the Soviet physicist Lev Landau in the 1930s.[4][5]
https://en.wikipedia.org/wiki/Quasiparticle
In thermodynamics, a critical point (or critical state) is the end point of a phase equilibrium curve. One example is the liquid–vapor critical point, the end point of the pressure–temperature curve that designates conditions under which a liquid and its vapor can coexist. At higher temperatures, the gas cannot be liquefied by pressure alone. At the critical point, defined by a critical temperature Tc and a critical pressure pc, phase boundaries vanish. Other examples include the liquid–liquid critical points in mixtures, and the ferromagnet–paramagnet transition (Curie temperature) in the absence of an external magnetic field.[2]
https://en.wikipedia.org/wiki/Critical_point_(thermodynamics)
In general relativity, a vacuum solution is a Lorentzian manifold whose Einstein tensor vanishes identically. According to the Einstein field equation, this means that the stress–energy tensor also vanishes identically, so that no matter or non-gravitational fields are present. These are distinct from the electrovacuum solutions, which take into account the electromagnetic field in addition to the gravitational field. Vacuum solutions are also distinct from the lambdavacuum solutions, where the only term in the stress–energy tensor is the cosmological constant term (and thus, the lambdavacuums can be taken as cosmological models).
More generally, a vacuum region in a Lorentzian manifold is a region in which the Einstein tensor vanishes.
Vacuum solutions are a special case of the more general exact solutions in general relativity.
https://en.wikipedia.org/wiki/Vacuum_solution_(general_relativity)
Now imagine a stack of hyperbolic disks where each disk represents the state of the universe at a given time. The resulting geometric object is three-dimensional anti-de Sitter space.[19] It looks like a solid cylinder in which any cross section is a copy of the hyperbolic disk. Time runs along the vertical direction in this picture. The surface of this cylinder plays an important role in the AdS/CFT correspondence. As with the hyperbolic plane, anti-de Sitter space is curved in such a way that any point in the interior is actually infinitely far from this boundary surface.[21]
This construction describes a hypothetical universe with only two space and one time dimension, but it can be generalized to any number of dimensions. Indeed, hyperbolic space can have more than two dimensions and one can "stack up" copies of hyperbolic space to get higher-dimensional models of anti-de Sitter space.[19]
https://en.wikipedia.org/wiki/AdS/CFT_correspondence
The most famous example of the AdS/CFT correspondence states that type IIB string theory on the product space is equivalent to N = 4 supersymmetric Yang–Mills theory on the four-dimensional boundary.[26] In this example, the spacetime on which the gravitational theory lives is effectively five-dimensional (hence the notation ), and there are five additional compact dimensions (encoded by the factor). In the real world, spacetime is four-dimensional, at least macroscopically, so this version of the correspondence does not provide a realistic model of gravity. Likewise, the dual theory is not a viable model of any real-world system as it assumes a large amount of supersymmetry. Nevertheless, as explained below, this boundary theory shares some features in common with quantum chromodynamics, the fundamental theory of the strong force. It describes particles similar to the gluons of quantum chromodynamics together with certain fermions.[8] As a result, it has found applications in nuclear physics, particularly in the study of the quark–gluon plasma.[27]
https://en.wikipedia.org/wiki/AdS/CFT_correspondence
Another realization of the correspondence states that M-theory on is equivalent to the so-called (2,0)-theory in six dimensions.[3] In this example, the spacetime of the gravitational theory is effectively seven-dimensional. The existence of the (2,0)-theory that appears on one side of the duality is predicted by the classification of superconformal field theories. It is still poorly understood because it is a quantum mechanical theory without a classical limit.[28] Despite the inherent difficulty in studying this theory, it is considered to be an interesting object for a variety of reasons, both physical and mathematical.[29]
Yet another realization of the correspondence states that M-theory on is equivalent to the ABJM superconformal field theory in three dimensions.[30] Here the gravitational theory has four noncompact dimensions, so this version of the correspondence provides a somewhat more realistic description of gravity.[31]
https://en.wikipedia.org/wiki/AdS/CFT_correspondence
Applications to quantum gravity
A non-perturbative formulation of string theory
In quantum field theory, one typically computes the probabilities of various physical events using the techniques of perturbation theory. Developed by Richard Feynman and others in the first half of the twentieth century, perturbative quantum field theory uses special diagrams called Feynman diagrams to organize computations. One imagines that these diagrams depict the paths of point-like particles and their interactions.[32] Although this formalism is extremely useful for making predictions, these predictions are only possible when the strength of the interactions, the coupling constant, is small enough to reliably describe the theory as being close to a theory without interactions.[33]
The starting point for string theory is the idea that the point-like particles of quantum field theory can also be modeled as one-dimensional objects called strings. The interaction of strings is most straightforwardly defined by generalizing the perturbation theory used in ordinary quantum field theory. At the level of Feynman diagrams, this means replacing the one-dimensional diagram representing the path of a point particle by a two-dimensional surface representing the motion of a string. Unlike in quantum field theory, string theory does not yet have a full non-perturbative definition, so many of the theoretical questions that physicists would like to answer remain out of reach.[34]
The problem of developing a non-perturbative formulation of string theory was one of the original motivations for studying the AdS/CFT correspondence.[35] As explained above, the correspondence provides several examples of quantum field theories which are equivalent to string theory on anti-de Sitter space. One can alternatively view this correspondence as providing a definition of string theory in the special case where the gravitational field is asymptotically anti-de Sitter (that is, when the gravitational field resembles that of anti-de Sitter space at spatial infinity). Physically interesting quantities in string theory are defined in terms of quantities in the dual quantum field theory.[20]
Black hole information paradox
In 1975, Stephen Hawking published a calculation which suggested that black holes are not completely black but emit a dim radiation due to quantum effects near the event horizon.[36] At first, Hawking's result posed a problem for theorists because it suggested that black holes destroy information. More precisely, Hawking's calculation seemed to conflict with one of the basic postulates of quantum mechanics, which states that physical systems evolve in time according to the Schrödinger equation. This property is usually referred to as unitarity of time evolution. The apparent contradiction between Hawking's calculation and the unitarity postulate of quantum mechanics came to be known as the black hole information paradox.[37]
The AdS/CFT correspondence resolves the black hole information paradox, at least to some extent, because it shows how a black hole can evolve in a manner consistent with quantum mechanics in some contexts. Indeed, one can consider black holes in the context of the AdS/CFT correspondence, and any such black hole corresponds to a configuration of particles on the boundary of anti-de Sitter space.[38] These particles obey the usual rules of quantum mechanics and in particular evolve in a unitary fashion, so the black hole must also evolve in a unitary fashion, respecting the principles of quantum mechanics.[39] In 2005, Hawking announced that the paradox had been settled in favor of information conservation by the AdS/CFT correspondence, and he suggested a concrete mechanism by which black holes might preserve information.[40]
Applications to quantum field theory
Nuclear physics
One physical system which has been studied using the AdS/CFT correspondence is the quark–gluon plasma, an exotic state of matter produced in particle accelerators. This state of matter arises for brief instants when heavy ions such as gold or lead nuclei are collided at high energies. Such collisions cause the quarks that make up atomic nuclei to deconfine at temperatures of approximately two trillion kelvins, conditions similar to those present at around 10−11 seconds after the Big Bang.[41]
The physics of the quark–gluon plasma is governed by quantum chromodynamics, but this theory is mathematically intractable in problems involving the quark–gluon plasma.[42] In an article appearing in 2005, Đàm Thanh Sơn and his collaborators showed that the AdS/CFT correspondence could be used to understand some aspects of the quark–gluon plasma by describing it in the language of string theory.[27] By applying the AdS/CFT correspondence, Sơn and his collaborators were able to describe the quark gluon plasma in terms of black holes in five-dimensional spacetime. The calculation showed that the ratio of two quantities associated with the quark–gluon plasma, the shear viscosity and volume density of entropy , should be approximately equal to a certain universal constant:
where denotes the reduced Planck constant and is the Boltzmann constant.[43] In addition, the authors conjectured that this universal constant provides a lower bound for in a large class of systems. In 2008, the predicted value of this ratio for the quark–gluon plasma was confirmed at the Relativistic Heavy Ion Collider at Brookhaven National Laboratory.[44]
Another important property of the quark–gluon plasma is that very high energy quarks moving through the plasma are stopped or "quenched" after traveling only a few femtometers. This phenomenon is characterized by a number called the jet quenching parameter, which relates the energy loss of such a quark to the squared distance traveled through the plasma. Calculations based on the AdS/CFT correspondence have allowed theorists to estimate , and the results agree roughly with the measured value of this parameter, suggesting that the AdS/CFT correspondence will be useful for developing a deeper understanding of this phenomenon.[45]
Condensed matter physics
Over the decades, experimental condensed matter physicists have discovered a number of exotic states of matter, including superconductors and superfluids. These states are described using the formalism of quantum field theory, but some phenomena are difficult to explain using standard field theoretic techniques. Some condensed matter theorists including Subir Sachdev hope that the AdS/CFT correspondence will make it possible to describe these systems in the language of string theory and learn more about their behavior.[47]
So far some success has been achieved in using string theory methods to describe the transition of a superfluid to an insulator. A superfluid is a system of electrically neutral atoms that flows without any friction. Such systems are often produced in the laboratory using liquid helium, but recently[when?] experimentalists have developed new ways of producing artificial superfluids by pouring trillions of cold atoms into a lattice of criss-crossing lasers. These atoms initially behave as a superfluid, but as experimentalists increase the intensity of the lasers, they become less mobile and then suddenly transition to an insulating state. During the transition, the atoms behave in an unusual way. For example, the atoms slow to a halt at a rate that depends on the temperature and on Planck's constant, the fundamental parameter of quantum mechanics, which does not enter into the description of the other phases. This behavior has recently been understood by considering a dual description where properties of the fluid are described in terms of a higher dimensional black hole.[48]
Criticism
With many physicists turning towards string-based methods to solve problems in nuclear and condensed matter physics, some theorists working in these areas have expressed doubts about whether the AdS/CFT correspondence can provide the tools needed to realistically model real-world systems. In a talk at the Quark Matter conference in 2006,[49] an American physicist, Larry McLerran pointed out that the N = 4 super Yang–Mills theory that appears in the AdS/CFT correspondence differs significantly from quantum chromodynamics, making it difficult to apply these methods to nuclear physics. According to McLerran,
supersymmetric Yang–Mills is not QCD ... It has no mass scale and is conformally invariant. It has no confinement and no running coupling constant. It is supersymmetric. It has no chiral symmetry breaking or mass generation. It has six scalar and fermions in the adjoint representation ... It may be possible to correct some or all of the above problems, or, for various physical problems, some of the objections may not be relevant. As yet there is not consensus nor compelling arguments for the conjectured fixes or phenomena which would insure that the supersymmetric Yang Mills results would reliably reflect QCD.[49]
In a letter to Physics Today, Nobel laureate Philip W. Anderson voiced similar concerns about applications of AdS/CFT to condensed matter physics, stating
As a very general problem with the AdS/CFT approach in condensed-matter theory, we can point to those telltale initials "CFT"—conformal field theory. Condensed-matter problems are, in general, neither relativistic nor conformal. Near a quantum critical point, both time and space may be scaling, but even there we still have a preferred coordinate system and, usually, a lattice. There is some evidence of other linear-T phases to the left of the strange metal about which they are welcome to speculate, but again in this case the condensed-matter problem is overdetermined by experimental facts.[50]
https://en.wikipedia.org/wiki/AdS/CFT_correspondence
Three-dimensional gravity
In order to better understand the quantum aspects of gravity in our four-dimensional universe, some physicists have considered a lower-dimensional mathematical model in which spacetime has only two spatial dimensions and one time dimension.[64] In this setting, the mathematics describing the gravitational field simplifies drastically, and one can study quantum gravity using familiar methods from quantum field theory, eliminating the need for string theory or other more radical approaches to quantum gravity in four dimensions.[65]
Beginning with the work of J. David Brown and Marc Henneaux in 1986,[66] physicists have noticed that quantum gravity in a three-dimensional spacetime is closely related to two-dimensional conformal field theory. In 1995, Henneaux and his coworkers explored this relationship in more detail, suggesting that three-dimensional gravity in anti-de Sitter space is equivalent to the conformal field theory known as Liouville field theory.[67] Another conjecture formulated by Edward Witten states that three-dimensional gravity in anti-de Sitter space is equivalent to a conformal field theory with monster group symmetry.[68] These conjectures provide examples of the AdS/CFT correspondence that do not require the full apparatus of string or M-theory.[69]
dS/CFT correspondence
Unlike our universe, which is now known to be expanding at an accelerating rate, anti-de Sitter space is neither expanding nor contracting. Instead it looks the same at all times.[19] In more technical language, one says that anti-de Sitter space corresponds to a universe with a negative cosmological constant, whereas the real universe has a small positive cosmological constant.[70]
Although the properties of gravity at short distances should be somewhat independent of the value of the cosmological constant,[71] it is desirable to have a version of the AdS/CFT correspondence for positive cosmological constant. In 2001, Andrew Strominger introduced a version of the duality called the dS/CFT correspondence.[72] This duality involves a model of spacetime called de Sitter space with a positive cosmological constant. Such a duality is interesting from the point of view of cosmology since many cosmologists believe that the very early universe was close to being de Sitter space.[19] Our universe may also resemble de Sitter space in the distant future.[19]
Kerr/CFT correspondence
Although the AdS/CFT correspondence is often useful for studying the properties of black holes,[73] most of the black holes considered in the context of AdS/CFT are physically unrealistic. Indeed, as explained above, most versions of the AdS/CFT correspondence involve higher-dimensional models of spacetime with unphysical supersymmetry.
In 2009, Monica Guica, Thomas Hartman, Wei Song, and Andrew Strominger showed that the ideas of AdS/CFT could nevertheless be used to understand certain astrophysical black holes. More precisely, their results apply to black holes that are approximated by extremal Kerr black holes, which have the largest possible angular momentum compatible with a given mass.[74] They showed that such black holes have an equivalent description in terms of conformal field theory. The Kerr/CFT correspondence was later extended to black holes with lower angular momentum.[75]
Higher spin gauge theories
The AdS/CFT correspondence is closely related to another duality conjectured by Igor Klebanov and Alexander Markovich Polyakov in 2002.[76] This duality states that certain "higher spin gauge theories" on anti-de Sitter space are equivalent to conformal field theories with O(N) symmetry. Here the theory in the bulk is a type of gauge theory describing particles of arbitrarily high spin. It is similar to string theory, where the excited modes of vibrating strings correspond to particles with higher spin, and it may help to better understand the string theoretic versions of AdS/CFT and possibly even prove the correspondence.[77] In 2010, Simone Giombi and Xi Yin obtained further evidence for this duality by computing quantities called three-point functions.[78]
See also
https://en.wikipedia.org/wiki/AdS/CFT_correspondence
| Chelidonura livida | |
|---|---|

| |
| A live individual of Chelidonura livida, in situ off Sharm el Sheik, Egypt | |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| (unranked): | |
| Superfamily: | |
| Family: | |
| Genus: | |
| Species: | C. livida
|
| Binomial name | |
| Chelidonura livida Yonow, 1994
| |
Chelidonura livida is a species of sea slug, or "headshield slug", a marine opisthobranch gastropod mollusk in the family Aglajidae.
https://en.wikipedia.org/wiki/Chelidonura_livida
Heavy fuel oil (HFO) is a category of fuel oils of a tar-like consistency. Also known as bunker fuel, or residual fuel oil, HFO is the result or remnant from the distillation and cracking process of petroleum. For this reason, HFO is contaminated with several different compounds including aromatics, sulfur and nitrogen, making emissions upon combustion more polluting compared to other fuel oils.[1] HFO is predominantly used as a fuel source for marine vessel propulsion due to its relatively low cost compared to cleaner fuel sources such as distillates.[2][3] The use and carriage of HFO on-board vessels presents several environmental concerns, namely the risk of oil spill and the emission of toxic compounds and particulates including black carbon. Presently, the use of HFOs is banned as a fuel source for ships travelling in the Antarctic as part of the International Maritime Organization's (IMO) International Code for Ships Operating in Polar Waters (Polar Code).[4] For similar reasons, an HFO ban in Arctic waters is currently being considered.[5]
https://en.wikipedia.org/wiki/Heavy_fuel_oil
The Kuwaiti oil fires were caused by the Iraqi military setting fire to a reported 605 to 732 oil wells along with an unspecified number of oil filled low-lying areas, such as oil lakes and fire trenches, as part of a scorched earth policy while retreating from Kuwait in 1991 due to the advances of US-led coalition forces in the Gulf War.[3] The fires were started in January and February 1991, and the first oil well fires were extinguished in early April 1991, with the last well capped on November 6, 1991.[4]
https://en.wikipedia.org/wiki/Kuwaiti_oil_fires
The Black Patch Tobacco Wars were a period of civil unrest and violence in the western counties of the U.S. states of Kentucky and Tennessee at the turn of the 20th century, circa 1904–1909. The so-called "Black Patch" consists of about 30 counties in southwestern Kentucky and northwestern Tennessee; during that period this area was the leading worldwide supplier of dark fired tobacco. It was so named for the wood smoke and fire-curing process which it undergoes after harvest. This type of tobacco is used primarily in snuff, chewing and pipe tobacco.[citation needed]
The primary antagonists were the American Tobacco Company (ATC) (owned by James B. Duke), historically one of the largest U.S. industrial monopolies, and the Dark Tobacco District Planters' Protective Association of Kentucky and Tennessee (PPA). This association of planters formed September 24, 1904 in protest of the monopoly ATC practice of paying deflated prices for their product and with the intent to control their own product and pricing by banding together.[citation needed]
The initial idea of the PPA was to "pool"[1] and withhold their tobacco until the ATC agreed to pay higher prices. When this plan was unsuccessful, many farmers resorted to violence and vigilante practices, organizing as the Silent Brigade or Night Riders. They committed numerous acts of violence and destroyed crops, machinery, livestock, and tobacco warehouses, even capturing whole towns. They raided Princeton, Hopkinsville, and Russellville, Kentucky, destroying tobacco warehouses.[citation needed]
https://en.wikipedia.org/wiki/Black_Patch_Tobacco_Wars
| First attested | November 1966 |
|---|---|
| Other name(s) | Winged Man, Bird Man, UFO-Bird, Mason Bird Monster |
| Country | United States |
| Region | Point Pleasant, West Virginia |
| Part of a series on the |
| Paranormal |
|---|
|
|
|
|
|
|
|
|
In West Virginia folklore, the Mothman is a humanoid creature reportedly seen in the Point Pleasant area from November 15, 1966, to December 15, 1967. The first newspaper report was published in the Point Pleasant Register, dated November 16, 1966, titled "Couples See Man-Sized Bird ... Creature ... Something".[1] The national press soon picked up the reports and helped spread the story across the United States. The source of the legend is believed to have originated from sightings of out-of-migration sandhill cranes or herons.[2][3]
The creature was introduced to a wider audience by Gray Barker in 1970,[4][5] and was later popularized by John Keel in his 1975 book The Mothman Prophecies,[6] claiming that there were supernatural events related to the sightings, and a connection to the collapse of the Silver Bridge. The book was later adapted into a 2002 film, starring Richard Gere.[7]
An annual festival in Point Pleasant is devoted to the Mothman legend.[8]
https://en.wikipedia.org/wiki/Mothman
Shark is the naming term of all members of Selachimorpha suborder in the subclass Elasmobranchii, in the class Chondrichthyes. The Elasmobranchii also include rays and skates; the Chondrichthyes also include Chimaeras. The first shark-like chondrichthyans appeared in the oceans 430 million years ago,[1] developing into the crown group of sharks by the Early Jurassic.[2]
Listed below are extant species of shark. Sharks are spread across 512 described and 23 undescribed species in eight orders. The families and genera within the orders are listed in alphabetical order. Also included is a field guide to place sharks into the correct order.
https://en.wikipedia.org/wiki/List_of_sharks
| Holothuria atra | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Echinodermata |
| Class: | Holothuroidea |
| Order: | Holothuriida |
| Family: | Holothuriidae |
| Genus: | Holothuria |
| Species: | H. atra
|
| Binomial name | |
| Holothuria atra Jaeger, 1833 [2]
| |
| Synonyms[2] | |
| |
Holothuria atra, commonly known as the black sea cucumber or lollyfish, is a species of marine invertebrate in the family Holothuriidae. It was placed in the subgenus Halodeima by Pearson in 1914, making its full scientific name Holothuria (Halodeima) atra. It is the type species of the subgenus.[2]
https://en.wikipedia.org/wiki/Holothuria_atra
Laverbread (/ˈleɪvər-, ˈlɑːvər-/; Welsh: bara lafwr or bara lawr; Irish: sleabhac) is a food product made from laver, an edible seaweed (littoral alga) consumed mainly in Wales as part of local traditional cuisine. The seaweed is commonly found around the west coast of Great Britain, and the coasts of Ireland, where it is known as sleabhac.[1] It is smooth in texture and forms delicate, sheetlike thalli, often clinging to rocks. The principal variety is Porphyra umbilicalis. Porphyra (laver seaweed) is classified as red algae; it tends to be a brownish colour, but boils down to a dark green pulp when prepared. Laver seaweed has a high content of dietary minerals, particularly iodine and iron. The high iodine content gives the seaweed a distinctive flavour in common with olives and oysters.
Laver seaweed has been cultivated as a food in Wales since at least the 17th century. It is prepared by repeated washings and then boiling until it becomes the soft purée-like product known as laverbread. The gelatinous paste that results can then be sold as it is or rolled in oatmeal. It is sometimes also coated with oatmeal prior to frying. Laverbread is traditionally eaten fried with bacon and cockles as part of a Welsh breakfast or, in the southwest of England, with hog's pudding.
https://en.wikipedia.org/wiki/Laverbread
The bat-eared fox (Otocyon megalotis) is a species of fox found on the African savanna. It is the only extant species of the genus Otocyon[1] and considered a basal canid species.[4] Fossil records indicate this canid first appeared during the middle Pleistocene.[5]
It is named for its large ears, which have a role in thermoregulation.[3] The bat referred to in its colloquial name is possibly the Egyptian slit-faced bat (Nycteris thebaica), which is abundant in the region and has very large ears.[6] Although not commonly used, other vernacular names include big-eared fox, black-eared fox, long-eared fox,[7] Delalande's fox, cape fox,[note 1][8] and motlosi.[3]
https://en.wikipedia.org/wiki/Bat-eared_fox
An active volcano is a volcano which is either erupting or is likely to erupt in the future. An active volcano which is not currently erupting is known as a dormant volcano.
https://en.wikipedia.org/wiki/Active_volcano
Schwarzschild wormholes
The first type of wormhole solution discovered was the Schwarzschild wormhole, which would be present in the Schwarzschild metric describing an eternal black hole, but it was found that it would collapse too quickly for anything to cross from one end to the other. Wormholes that could be crossed in both directions, known as traversable wormholes, were thought to be possible only if exotic matter with negative energy density could be used to stabilize them.[12] However, physicists later reported that microscopic traversable wormholes may be possible and not require any exotic matter, instead requiring only electrically charged fermionic matter with small enough mass that it cannot collapse into a charged black hole.[13][14] While such wormholes, if possible, may be limited to transfers of information, humanly traversable wormholes may exist if reality can broadly be described by the Randall–Sundrum model 2, a brane-based theory consistent with string theory.[15][16]
https://en.wikipedia.org/wiki/Wormhole#Schwarzschild_wormholes
Roughcast or pebbledash is a coarse plaster surface used on outside walls that consists of lime and sometimes cement mixed with sand, small gravel and often pebbles or shells.[1] The materials are mixed into a slurry and are then thrown at the working surface with a trowel or scoop. The idea is to maintain an even spread, free from lumps, ridges or runs and without missing any background. Roughcasting incorporates the stones in the mix, whereas pebbledashing adds them on top.
According to the Encyclopædia Britannica Eleventh Edition (1910–1911), roughcast had been a widespread exterior coating given to the walls of common dwellings and outbuildings, but it was then frequently employed for decorative effect on country houses, especially those built using timber framing (half timber). Variety can be obtained on the surface of the wall by small pebbles of different colours, and in the Tudor period fragments of glass were sometimes embedded.[2]
https://en.wikipedia.org/wiki/Roughcast
| Southern stargazer | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Actinopterygii |
| Order: | Trachiniformes |
| Family: | Uranoscopidae |
| Genus: | Astroscopus |
| Species: | A. y-graecum
|
| Binomial name | |
| Astroscopus y-graecum (Cuvier, 1829)
| |
| Synonyms[2] | |
|
Uranoscopus y-graecum Cuvier, 1829 | |
The southern stargazer (Astroscopus y-graecum) is a species of marine fish in the family Uranoscopidae and genus Astroscopus. They are native to the United States.
https://en.wikipedia.org/wiki/Southern_stargazer
| UTAS UTS-15 | |
|---|---|
 The UTAS UTS-15 | |
| Type | Pump-action shotgun |
| Place of origin | Turkey |
| Production history | |
| Designed | 2006–2012 |
| Manufacturer | UTAS |
| Produced | 2012–present |
| Variants | UTS-15 Desert UTS-15 Marine UTS-15 Hunting |
| Specifications | |
| Mass | 6.9 lb (3.1 kg) empty |
| Length | 28.3 in (72 cm) |
| Barrel length | 18.5 in (47 cm) |
| Cartridge | 12 gauge |
| Action | Pump-action |
| Feed system | Dual, selectable 7-round tube magazines |
| Sights | Picatinny rail provided for optics |
The UTAS UTS-15 is a 12 gauge pump-action shotgun with two 7-round magazine tubes that can feed in an alternating or selecting pattern. The UTS-15 has a 28.3" overall length with an 18.5" barrel, chambered for 2½", 2¾", and 3" magnum ammunition. Constructed primarily of fiber reinforced injection molded polymer, the UTS-15 weighs 6.9 lbs. Additionally, there is a top mounted picatinny rail for the mounting of a wide variety of both iron and optical sights, coupled with Beretta style barrel threading for choke tubes.[1]
https://en.wikipedia.org/wiki/UTAS_UTS-15
| Golden moles[2] Temporal range:
| |
|---|---|
| Cape golden mole | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Afrosoricida |
| Suborder: | Chrysochloridea Broom, 1915 |
| Family: | Chrysochloridae Gray, 1825 |
| Type genus | |
| Chrysochloris Lacépède, 1799
| |
| Genera | |
| |
Golden moles are small insectivorous burrowing mammals endemic to Sub-Saharan Africa. They comprise the family Chrysochloridae and as such they are taxonomically distinct from the true moles, family Talpidae, and other mole-like families, all of which, to various degrees, they resemble as a result of evolutionary convergence. There are 21 species. Some (e.g., Chrysochloris asiatica, Amblysomus hottentotus) are relatively common, whereas others (e.g., species of Chrysospalax, Cryptochloris, Neamblysomus) are rare and endangered.
https://en.wikipedia.org/wiki/Golden_mole
| Hyperprosopon argenteum | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Actinopterygii |
| Family: | Embiotocidae |
| Genus: | Hyperprosopon |
| Species: | H. argenteum
|
| Binomial name | |
| Hyperprosopon argenteum (Gibbons, 1854)
| |
| Synonyms | |
| |
Hyperprosopon argenteum, the walleye surfperch, is a species of surfperch native to the eastern Pacific Ocean.
https://en.wikipedia.org/wiki/Hyperprosopon_argenteum
Mare Boreum quadrangle
 Map of Mare Boreum quadrangle from Mars Orbiter Laser Altimeter (MOLA) data. The highest elevations are red and the lowest are blue. | |
| Coordinates | 75°N 0°ECoordinates: 75°N 0°E |
|---|---|
The Mare Boreum quadrangle is one of a series of 30 quadrangle maps of Mars used by the United States Geological Survey (USGS) Astrogeology Research Program. The Mare Boreum quadrangle is also referred to as MC-1 (Mars Chart-1).[1] Its name derives from an older name for a feature that is now called Planum Boreum, a large plain surrounding the polar cap.[2]
The quadrangle covers all of the Martian surface north of latitude 65°. It includes the north polar ice cap, which has a swirl pattern and is roughly 1,100 kilometres (680 miles) across. Mariner 9 in 1972 discovered a belt of sand dunes that ring the polar ice deposits, which is 500 kilometres (310 miles) across in some places and may be the largest dune field in the Solar System.[3] The ice cap is surrounded by the vast plains of Planum Boreum and Vastitas Borealis. Close to the pole, there is a large valley, Chasma Boreale, that may have been formed from water melting from the ice cap.[4] An alternative view is that it was made by winds coming off the cold pole.[5][6] Another prominent feature is a smooth rise, formerly called Olympia Planitia. In the summer, a dark collar around the residual cap becomes visible; it is mostly caused by dunes.[7] The quadrangle includes some very large craters that stand out in the north because the area is smooth with little change in topography. These large craters are Lomonosov and Korolev. Although smaller, the crater Stokes is also prominent.
The Phoenix lander landed on Vastitas Borealis within the Mare Boreum quadrangle at 68.218830° N and 234.250778° E on May 25, 2008.[8] The probe collected and analyzed soil samples in an effort to detect water and determine how hospitable the planet might once have been for life to grow. It remained active there until winter conditions became too harsh around five months later.[9]
After the mission ended the journal Science reported that chloride, bicarbonate, magnesium, sodium potassium, calcium, and possibly sulfate were detected in the samples analyzed by Phoenix. The pH was narrowed down to 7.7±0.5. Perchlorate (ClO4), a strong oxidizer at elevated temperatures, was detected. This was a significant discovery because the chemical has the potential of being used for rocket fuel and as a source of oxygen for future colonists. Also, under certain conditions perchlorate can inhibit life; however some microorganisms obtain energy from the substance (by anaerobic reduction). The chemical when mixed with water can greatly lower freezing points, in a manner similar to how salt is applied to roads to melt ice. So, perchlorate may be allowing small amounts of liquid water to form on Mars today. Gullies, which are common in certain areas of Mars, may have formed from perchlorate melting ice and causing water to erode soil on steep slopes.[10]
Much direct evidence was found for water at this location.[11]
Freezing of atmosphere
Research based on slight changes in the orbits of spacecraft around Mars over 16 years found that when one hemisphere experiences winter, approximately 3 trillion to 4 trillion tons of carbon dioxide freezes out of the atmosphere onto the northern and southern polar caps. This represents 12 to 16 percent of the mass of the entire Martian atmosphere. These observation support predictions from the Mars Global Reference Atmospheric Model—2010.[12][13]
Proof for ocean
Strong evidence for a one time ancient ocean was found in Mare Boreum near the north pole (as well as the south pole). In March 2015, a team of scientists published results showing that this region was highly enriched with deuterium, heavy hydrogen, by seven times as much as the Earth. This means that Mars has lost a volume of water 6.5 times what is stored in today's polar caps. The water for a time would have formed an ocean in the low-lying Mare Boreum. The amount of water could have covered the planet about 140 meters, but was probably in an ocean that in places would be almost 1 mile deep.
This international team used ESO's Very Large Telescope, along with instruments at the W. M. Keck Observatory and the NASA Infrared Telescope Facility, to map out different forms of water in Mars's atmosphere over a six-year period.[14][15]
Ice cap
From observations with the Shallow Radar instrument (SHARAD) onboard the Mars Reconnaissance Orbiter, researchers determined that the total volume of water ice in the northern ice cap is 821000 cubic kilometers. That is equal to 30% of the Earth's Greenland ice sheet, or enough to cover the surface of Mars to a depth of 5.6 meters[16][17][18]
Layers exposed in northern ice cap, as seen by HiRISE under HiWish program
Ridges
Dunes
Sand dunes have been found in many places on Mars. The presence of dunes shows that the planet has an atmosphere with wind, for dunes require wind to pile up the sand. Most dunes on Mars are black because of the weathering of the volcanic rock basalt.[19][7] Black sand can be found on Earth on Hawaii and on some tropical South Pacific islands.[20] Sand is common on Mars due to the old age of the surface that has allowed rocks to erode into sand. Dunes on Mars have been observed to move many meters.[21][22] In this process, sand moves up the windward side and then falls down the leeward side of the dune, thus caused the dune to go toward the leeward side (or slip face).[23] When images are enlarged, some dunes on Mars display ripples on their surfaces.[24] These are caused by sand grains rolling and bouncing up the windward surface of a dune. The bouncing grains tend to land on the windward side of each ripple. The grains do not bounce very high so it does not take much to stop them.
Defrosting dunes and ice in troughs of polygons, as seen by HiRISE under HiWish program
Defrosting surface, as seen by HiRISE under HiWish program Frost is disappearing in patches from a dune and from the surrounding surface. The trough boundaries around the polygon shapes still contain frost; hence they are white. Note: the north side (side near top) has not defrosted because the sun is coming from the other side.
Other Mars Quadrangles
Interactive Mars map

See also
References
- "PIA03467: The MGS MOC Wide Angle Map of Mars". Photojournal. NASA / Jet Propulsion Laboratory. February 16, 2002. Retrieved December 16, 2012.
External links
https://en.wikipedia.org/wiki/Mare_Boreum_quadrangle
| Megapode | |
|---|---|

| |
| Australian brushturkey (Alectura lathami) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Aves |
| Order: | Galliformes |
| Family: | Megapodiidae Lesson, 1831 |
| Genera | |
The megapodes, also known as incubator birds or mound-builders, are stocky, medium-large, chicken-like birds with small heads and large feet in the family Megapodiidae. Their name literally means "large foot" and is a reference to the heavy legs and feet typical of these terrestrial birds. All are browsers, and all but the malleefowl occupy wooded habitats. Most are brown or black in color. Megapodes are superprecocial, hatching from their eggs in the most mature condition of any bird. They hatch with open eyes, bodily coordination and strength, full wing feathers, and downy body feathers, and are able to run, pursue prey, and in some species, fly on the same day they hatch.[1]
https://en.wikipedia.org/wiki/Megapode
| Literature | ||||||
|---|---|---|---|---|---|---|
 | ||||||
| Major forms | ||||||
| Prose genres | ||||||
|
||||||
| Poetry genres | ||||||
|
||||||
| Dramatic genres | ||||||
| History and lists | ||||||
| Discussion | ||||||
| Topics | ||||||
|
| ||||||
Literature is any collection of written work, but it is also used more narrowly for writings specifically considered to be an art form, especially prose fiction, drama, and poetry.[1] In recent centuries, the definition has expanded to include oral literature, much of which has been transcribed.[2][3] Literature is a method of recording, preserving, and transmitting knowledge and entertainment, and can also have a social, psychological, spiritual, or political role.
Literature, as an art form, can also include works in various non-fiction genres, such as biography, diaries, memoir, letters, and essays. Within its broad definition, literature includes non-fictional books, articles or other printed information on a particular subject.[4][5]
Etymologically, the term derives from Latin literatura/litteratura "learning, a writing, grammar," originally "writing formed with letters," from litera/littera "letter".[6] In spite of this, the term has also been applied to spoken or sung texts.[7][8] Literature is often referred to synecdochically as "writing," and poetically as "the craft of writing" or simply "the craft." Syd Field described his discipline, screenwriting, as "a craft that occasionally rises to the level of art."[9]
Developments in print technology have allowed an ever-growing distribution and proliferation of written works, which now includes electronic literature.
https://en.wikipedia.org/wiki/Literature
| Nucras tessellata | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Reptilia |
| Order: | Squamata |
| Family: | Lacertidae |
| Genus: | Nucras |
| Species: | N. tessellata
|
| Binomial name | |
| Nucras tessellata (Smith, 1838)
| |
Nucras tessellata, also known as the western sandveld lizard, striped sandveld lizard, tiger lizard, striped sand lizard or banded sand lizard, is a species of lizard in the family Lacertidae.[1][2] It is native to western Southern Africa and is found in western South Africa (Western Cape and Northern Cape provinces), southwestern Botswana, and southern and central Namibia north to Khomas Highland. Its range includes several protected areas, including Tankwa Karoo National Park and ǀAi-ǀAis/Richtersveld Transfrontier Park.[1]
https://en.wikipedia.org/wiki/Nucras_tessellata
| Kalij pheasant | |
|---|---|

| |
| Male L. leucomelanos hamiltoni, Uttarakhand, India | |

| |
| Female L. leucomelanos hamiltoni, Uttarakhand, India | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Aves |
| Order: | Galliformes |
| Family: | Phasianidae |
| Genus: | Lophura |
| Species: | L. leucomelanos
|
| Binomial name | |
| Lophura leucomelanos (Latham, 1790)
| |
The kalij pheasant (Lophura leucomelanos) is a pheasant found in forests and thickets, especially in the Himalayan foothills, from Nepal, Pakistan to western Thailand. Males are rather variable depending on the subspecies involved, but all have at least partially glossy bluish-black plumage, while females are overall brownish. Both sexes have a bare red face and greyish legs (the latter separating it from the red-legged silver pheasant).[2] It is generally common and widespread, though three of its eastern subspecies (L. l. oatesi, L. l. lineata, and L. l. crawfurdi) are considered threatened and L. l. moffitti is virtually unknown in the wild.[2] On 21 October 2021, the Government of Jammu and Kashmir declared Kalij Pheasant as bird of the Union Territory of Jammu and Kashmir.[3]
The name is also spelled kaleege in old texts, such as Game Birds of India and Asia by Frank Finn,[4] though no longer in his Indian Sporting Birds.[5] The species was introduced to Hawaii[1] in 1962 as a gamebird.[6]
https://en.wikipedia.org/wiki/Kalij_pheasant
Physical properties of soil
The physical properties of soil, in order of decreasing importance for ecosystem services such as crop production, are texture, structure, bulk density, porosity, consistency, temperature, colour and resistivity.[1] Soil texture is determined by the relative proportion of the three kinds of soil mineral particles, called soil separates: sand, silt, and clay. At the next larger scale, soil structures called peds or more commonly soil aggregates are created from the soil separates when iron oxides, carbonates, clay, silica and humus, coat particles and cause them to adhere into larger, relatively stable secondary structures.[2] Soil bulk density, when determined at standardized moisture conditions, is an estimate of soil compaction.[3] Soil porosity consists of the void part of the soil volume and is occupied by gases or water. Soil consistency is the ability of soil materials to stick together. Soil temperature and colour are self-defining. Resistivity refers to the resistance to conduction of electric currents and affects the rate of corrosion of metal and concrete structures which are buried in soil.[4] These properties vary through the depth of a soil profile, i.e. through soil horizons. Most of these properties determine the aeration of the soil and the ability of water to infiltrate and to be held within the soil.[5]
| Property/behavior | Sand | Silt | Clay |
|---|---|---|---|
| Water-holding capacity | Low | Medium to high | High |
| Aeration | Good | Medium | Poor |
| Drainage rate | High | Slow to medium | Very slow |
| Soil organic matter level | Low | Medium to high | High to medium |
| Decomposition of organic matter | Rapid | Medium | Slow |
| Warm-up in spring | Rapid | Moderate | Slow |
| Compactability | Low | Medium | High |
| Susceptibility to wind erosion | Moderate (High if fine sand) | High | Low |
| Susceptibility to water erosion | Low (unless fine sand) | High | Low if aggregated, otherwise high |
| Shrink/Swell Potential | Very Low | Low | Moderate to very high |
| Sealing of ponds, dams, and landfills | Poor | Poor | Good |
| Suitability for tillage after rain | Good | Medium | Poor |
| Pollutant leaching potential | High | Medium | Low (unless cracked) |
| Ability to store plant nutrients | Poor | Medium to High | High |
| Resistance to pH change | Low | Medium | High |
Texture
The mineral components of soil are sand, silt and clay, and their relative proportions determine a soil's texture. Properties that are influenced by soil texture include porosity, permeability, infiltration, shrink-swell rate, water-holding capacity, and susceptibility to erosion. In the illustrated USDA textural classification triangle, the only soil in which neither sand, silt nor clay predominates is called loam. While even pure sand, silt or clay may be considered a soil, from the perspective of conventional agriculture a loam soil with a small amount of organic material is considered "ideal", inasmuch as fertilizers or manure are currently used to mitigate nutrient losses due to crop yields in the long term.[7] The mineral constituents of a loam soil might be 40% sand, 40% silt and the balance 20% clay by weight. Soil texture affects soil behaviour, in particular, its retention capacity for nutrients (e.g., cation exchange capacity)[8] and water.
Sand and silt are the products of physical and chemical weathering of the parent rock;[9] clay, on the other hand, is most often the product of the precipitation of the dissolved parent rock as a secondary mineral, except when derived from the weathering of mica.[10] It is the surface area to volume ratio (specific surface area) of soil particles and the unbalanced ionic electric charges within those that determine their role in the fertility of soil, as measured by its cation exchange capacity.[11][12] Sand is least active, having the least specific surface area, followed by silt; clay is the most active. Sand's greatest benefit to soil is that it resists compaction and increases soil porosity, although this property stands only for pure sand, not for sand mixed with smaller minerals which fill the voids among sand grains.[13] Silt is mineralogically like sand but with its higher specific surface area it is more chemically and physically active than sand. But it is the clay content of soil, with its very high specific surface area and generally large number of negative charges, that gives a soil its high retention capacity for water and nutrients.[11] Clay soils also resist wind and water erosion better than silty and sandy soils, as the particles bond tightly to each other,[14] and that with a strong mitigation effect of organic matter.[15]
Sand is the most stable of the mineral components of soil; it consists of rock fragments, primarily quartz particles, ranging in size from 2.0 to 0.05 mm (0.0787 to 0.0020 in) in diameter. Silt ranges in size from 0.05 to 0.002 mm (0.001969 to 7.9×10−5 in). Clay cannot be resolved by optical microscopes as its particles are 0.002 mm (7.9×10−5 in) or less in diameter and a thickness of only 10 angstroms (10−10 m).[16][17] In medium-textured soils, clay is often washed downward through the soil profile (a process called eluviation) and accumulates in the subsoil (a process called illuviation). There is no clear relationship between the size of soil mineral components and their mineralogical nature: sand and silt particles can be calcareous as well as siliceous,[18] while textural clay (0.002 mm (7.9×10−5 in)) can be made of very fine quartz particles as well as of multi-layered secondary minerals.[19] Soil mineral components belonging to a given textural class may thus share properties linked to their specific surface area (e.g. moisture retention) but not those linked to their chemical composition (e.g. cation exchange capacity).
Soil components larger than 2.0 mm (0.079 in) are classed as rock and gravel and are removed before determining the percentages of the remaining components and the textural class of the soil, but are included in the name. For example, a sandy loam soil with 20% gravel would be called gravelly sandy loam.
When the organic component of a soil is substantial, the soil is called organic soil rather than mineral soil. A soil is called organic if:
- Mineral fraction is 0% clay and organic matter is 20% or more
- Mineral fraction is 0% to 50% clay and organic matter is between 20% and 30%
- Mineral fraction is 50% or more clay and organic matter 30% or more.[20]
Structure
The clumping of the soil textural components of sand, silt and clay causes aggregates to form and the further association of those aggregates into larger units creates soil structures called peds (a contraction of the word pedolith). The adhesion of the soil textural components by organic substances, iron oxides, carbonates, clays, and silica, the breakage of those aggregates from expansion-contraction caused by freezing-thawing and wetting-drying cycles,[21] and the build-up of aggregates by soil animals, microbial colonies and root tips[22] shape soil into distinct geometric forms.[23][24] The peds evolve into units which have various shapes, sizes and degrees of development.[25] A soil clod, however, is not a ped but rather a mass of soil that results from mechanical disturbance of the soil such as cultivation. Soil structure affects aeration, water movement, conduction of heat, plant root growth and resistance to erosion.[26] Water, in turn, has a strong effect on soil structure, directly via the dissolution and precipitation of minerals, the mechanical destruction of aggregates (slaking)[27] and indirectly by promoting plant, animal and microbial growth.
Soil structure often gives clues to its texture, organic matter content, biological activity, past soil evolution, human use, and the chemical and mineralogical conditions under which the soil formed. While texture is defined by the mineral component of a soil and is an innate property of the soil that does not change with agricultural activities, soil structure can be improved or destroyed by the choice and timing of farming practices.[23]
Soil structural classes:[28]
- Types: Shape and arrangement of peds
- Platy: Peds are flattened one atop the other 1–10 mm thick. Found in the A-horizon of forest soils and lake sedimentation.
- Prismatic and Columnar: Prismlike peds are long in the vertical dimension, 10–100 mm wide. Prismatic peds have flat tops, columnar peds have rounded tops. Tend to form in the B-horizon in high sodium soil where clay has accumulated.
- Angular and subangular: Blocky peds are imperfect cubes, 5–50 mm, angular have sharp edges, subangular have rounded edges. Tend to form in the B-horizon where clay has accumulated and indicate poor water penetration.
- Granular and Crumb: Spheroid peds of polyhedrons, 1–10 mm, often found in the A-horizon in the presence of organic material. Crumb peds are more porous and are considered ideal.
- Classes: Size of peds whose ranges depend upon the above type
- Very fine or very thin: <1 mm platy and spherical; <5 mm blocky; <10 mm prismlike.
- Fine or thin: 1–2 mm platy, and spherical; 5–10 mm blocky; 10–20 mm prismlike.
- Medium: 2–5 mm platy, granular; 10–20 mm blocky; 20–50 prismlike.
- Coarse or thick: 5–10 mm platy, granular; 20–50 mm blocky; 50–100 mm prismlike.
- Very coarse or very thick: >10 mm platy, granular; >50 mm blocky; >100 mm prismlike.
- Grades: Is a measure of the degree of development or cementation within the peds that results in their strength and stability.
- Weak: Weak cementation allows peds to fall apart into the three textural constituents, sand, silt and clay.
- Moderate: Peds are not distinct in undisturbed soil but when removed they break into aggregates, some broken aggregates and little unaggregated material. This is considered ideal.
- Strong:Peds are distinct before removed from the profile and do not break apart easily.
- Structureless: Soil is entirely cemented together in one great mass such as slabs of clay or no cementation at all such as with sand.
At the largest scale, the forces that shape a soil's structure result from swelling and shrinkage that initially tend to act horizontally, causing vertically oriented prismatic peds. This mechanical process is mainly exemplified in the development of vertisols.[29] Clayey soil, due to its differential drying rate with respect to the surface, will induce horizontal cracks, reducing columns to blocky peds.[30] Roots, rodents, worms, and freezing-thawing cycles further break the peds into smaller peds of a more or less spherical shape.[22]
At a smaller scale, plant roots extend into voids (macropores) and remove water[31] causing macroporosity to increase and microporosity to decrease,[32] thereby decreasing aggregate size.[33] At the same time, root hairs and fungal hyphae create microscopic tunnels (micropores) that break up peds.[34][35]
At an even smaller scale, soil aggregation continues as bacteria and fungi exude sticky polysaccharides which bind soil into smaller peds.[36] The addition of the raw organic matter that bacteria and fungi feed upon encourages the formation of this desirable soil structure.[37]
At the lowest scale, the soil chemistry affects the aggregation or dispersal of soil particles. The clay particles contain polyvalent cations, such as aluminium, which give the faces of clay layers localized negative charges.[38] At the same time, the edges of the clay plates have a slight positive charge, due to the sorption of aluminium from the soil solution to exposed hydroxyl groups, thereby allowing the edges to adhere to the negative charges on the faces of other clay particles or to flocculate (form clumps).[39] On the other hand, when monovalent ions, such as sodium, invade and displace the polyvalent cations (single displacement reaction), they weaken the positive charges on the edges, while the negative surface charges are relatively strengthened. This leaves negative charge on the clay faces that repel other clay, causing the particles to push apart, and by doing so deflocculate clay suspensions.[40] As a result, the clay disperses and settles into voids between peds, causing those to close. In this way the open structure of the soil is destroyed and the soil is made impenetrable to air and water.[41] Such sodic soil (also called haline soil) tends to form columnar peds near the surface.[42]
Density
| Soil treatment and identification | Bulk density (g/cm3) | Pore space (%) |
|---|---|---|
| Tilled surface soil of a cotton field | 1.3 | 51 |
| Trafficked inter-rows where wheels passed surface | 1.67 | 37 |
| Traffic pan at 25 cm deep | 1.7 | 36 |
| Undisturbed soil below traffic pan, clay loam | 1.5 | 43 |
| Rocky silt loam soil under aspen forest | 1.62 | 40 |
| Loamy sand surface soil | 1.5 | 43 |
| Decomposed peat | 0.55 | 65 |
Soil particle density is typically 2.60 to 2.75 grams per cm3 and is usually unchanging for a given soil.[44] Soil particle density is lower for soils with high organic matter content,[45] and is higher for soils with high iron-oxides content.[46] Soil bulk density is equal to the dry mass of the soil divided by the volume of the soil; i.e., it includes air space and organic materials of the soil volume. Thereby soil bulk density is always less than soil particle density and is a good indicator of soil compaction.[47] The soil bulk density of cultivated loam is about 1.1 to 1.4 g/cm3 (for comparison water is 1.0 g/cm3).[48] Contrary to particle density, soil bulk density is highly variable for a given soil, with a strong causal relationship with soil biological activity and management strategies.[49] However, it has been shown that, depending on species and the size of their aggregates (faeces), earthworms may either increase or decrease soil bulk density.[50] A lower bulk density by itself does not indicate suitability for plant growth due to the confounding influence of soil texture and structure.[51] A high bulk density is indicative of either soil compaction or a mixture of soil textural classes in which small particles fill the voids among coarser particles.[52] Hence the positive correlation between the fractal dimension of soil, considered as a porous medium, and its bulk density,[53] that explains the poor hydraulic conductivity of silty clay loam in the absence of a faunal structure.[54]
Porosity
Pore space is that part of the bulk volume of soil that is not occupied by either mineral or organic matter but is open space occupied by either gases or water. In a productive, medium-textured soil the total pore space is typically about 50% of the soil volume.[55] Pore size varies considerably; the smallest pores (cryptopores; <0.1 μm) hold water too tightly for use by plant roots; plant-available water is held in ultramicropores, micropores and mesopores (0.1–75 μm); and macropores (>75 μm) are generally air-filled when the soil is at field capacity.
Soil texture determines total volume of the smallest pores;[56] clay soils have smaller pores, but more total pore space than sands,[57] despite of a much lower permeability.[58] Soil structure has a strong influence on the larger pores that affect soil aeration, water infiltration and drainage.[59] Tillage has the short-term benefit of temporarily increasing the number of pores of largest size, but these can be rapidly degraded by the destruction of soil aggregation.[60]
The pore size distribution affects the ability of plants and other organisms to access water and oxygen; large, continuous pores allow rapid transmission of air, water and dissolved nutrients through soil, and small pores store water between rainfall or irrigation events.[61] Pore size variation also compartmentalizes the soil pore space such that many microbial and faunal organisms are not in direct competition with one another, which may explain not only the large number of species present, but the fact that functionally redundant organisms (organisms with the same ecological niche) can co-exist within the same soil.[62]
Consistency
Consistency is the ability of soil to stick to itself or to other objects (cohesion and adhesion, respectively) and its ability to resist deformation and rupture. It is of approximate use in predicting cultivation problems[63] and the engineering of foundations.[64] Consistency is measured at three moisture conditions: air-dry, moist, and wet.[65] In those conditions the consistency quality depends upon the clay content. In the wet state, the two qualities of stickiness and plasticity are assessed. A soil's resistance to fragmentation and crumbling is assessed in the dry state by rubbing the sample. Its resistance to shearing forces is assessed in the moist state by thumb and finger pressure. Additionally, the cemented consistency depends on cementation by substances other than clay, such as calcium carbonate, silica, oxides and salts; moisture content has little effect on its assessment. The measures of consistency border on subjective compared to other measures such as pH, since they employ the apparent feel of the soil in those states.
The terms used to describe the soil consistency in three moisture states and a last not affected by the amount of moisture are as follows:
- Consistency of Dry Soil: loose, soft, slightly hard, hard, very hard, extremely hard
- Consistency of Moist Soil: loose, very friable, friable, firm, very firm, extremely firm
- Consistency of Wet Soil: nonsticky, slightly sticky, sticky, very sticky; nonplastic, slightly plastic, plastic, very plastic
- Consistency of Cemented Soil: weakly cemented, strongly cemented, indurated (requires hammer blows to break up)[66]
Soil consistency is useful in estimating the ability of soil to support buildings and roads. More precise measures of soil strength are often made prior to construction.[67]
Temperature
Soil temperature depends on the ratio of the energy absorbed to that lost.[68] Soil has a mean annual temperature from -10 to 26 °C according to biomes.[69] Soil temperature regulates seed germination,[70] breaking of seed dormancy,[71][72] plant and root growth[73] and the availability of nutrients.[74] Soil temperature has important seasonal, monthly and daily variations, fluctuations in soil temperature being much lower with increasing soil depth.[75] Heavy mulching (a type of soil cover) can slow the warming of soil in summer, and, at the same time, reduce fluctuations in surface temperature.[76]
Most often, agricultural activities must adapt to soil temperatures by:
- maximizing germination and growth by timing of planting (also determined by photoperiod)[77]
- optimizing use of anhydrous ammonia by applying to soil below 10 °C (50 °F)[78]
- preventing heaving and thawing due to frosts from damaging shallow-rooted crops[79]
- preventing damage to desirable soil structure by freezing of saturated soils[80]
- improving uptake of phosphorus by plants[81]
Soil temperatures can be raised by drying soils[82] or the use of clear plastic mulches.[83] Organic mulches slow the warming of the soil.[76]
There are various factors that affect soil temperature, such as water content,[84] soil color,[85] and relief (slope, orientation, and elevation),[86] and soil cover (shading and insulation), in addition to air temperature.[87] The color of the ground cover and its insulating properties have a strong influence on soil temperature.[88] Whiter soil tends to have a higher albedo than blacker soil cover, which encourages whiter soils to have lower soil temperatures.[85] The specific heat of soil is the energy required to raise the temperature of soil by 1 °C. The specific heat of soil increases as water content increases, since the heat capacity of water is greater than that of dry soil.[89] The specific heat of pure water is ~ 1 calorie per gram, the specific heat of dry soil is ~ 0.2 calories per gram, hence, the specific heat of wet soil is ~ 0.2 to 1 calories per gram (0.8 to 4.2 kJ per kilogram).[90] Also, a tremendous energy (~584 cal/g or 2442 kJ/kg at 25 °C) is required to evaporate water (known as the heat of vaporization). As such, wet soil usually warms more slowly than dry soil – wet surface soil is typically 3 to 6 °C colder than dry surface soil.[91]
Soil heat flux refers to the rate at which heat energy moves through the soil in response to a temperature difference between two points in the soil. The heat flux density is the amount of energy that flows through soil per unit area per unit time and has both magnitude and direction. For the simple case of conduction into or out of the soil in the vertical direction, which is most often applicable the heat flux density is:
In SI units
- is the heat flux density, in SI the units are W·m−2
- is the soils' conductivity, W·m−1·K−1. The thermal conductivity is sometimes a constant, otherwise an average value of conductivity for the soil condition between the surface and the point at depth is used.
- is the temperature difference (temperature gradient) between the two points in the soil between which the heat flux density is to be calculated. In SI the units are kelvin, K.
- is the distance between the two points within the soil, at which the temperatures are measured and between which the heat flux density is being calculated. In SI the units are meters m, and where x is measured positive downward.
Heat flux is in the direction opposite the temperature gradient, hence the minus sign. That is to say, if the temperature of the surface is higher than at depth x, the negative sign will result in a positive value for the heat flux q, and which is interpreted as the heat being conducted into the soil.
| Component | Thermal Conductivity (W·m‐1·K‐1) |
|---|---|
| Quartz | 8.8 |
| Clay | 2.9 |
| Organic matter | 0.25 |
| Water | 0.57 |
| Ice | 2.4 |
| Air | 0.025 |
| Dry soil | 0.2‐0.4 |
| Wet soil | 1–3 |
(Source[6])
Soil temperature is important for the survival and early growth of seedlings.[92] Soil temperatures affect the anatomical and morphological character of root systems.[93] All physical, chemical, and biological processes in soil and roots are affected in particular because of the increased viscosities of water and protoplasm at low temperatures.[94] In general, climates that do not preclude survival and growth of white spruce above ground are sufficiently benign to provide soil temperatures able to maintain white spruce root systems. In some northwestern parts of the range, white spruce occurs on permafrost sites[95] and although young unlignified roots of conifers may have little resistance to freezing,[96] the root system of containerized white spruce was not affected by exposure to a temperature of 5 to 20 °C.[97]
Optimum temperatures for tree root growth range between 10 °C and 25 °C in general[98] and for spruce in particular.[99] In 2-week-old white spruce seedlings that were then grown for 6 weeks in soil at temperatures of 15 °C, 19 °C, 23 °C, 27 °C, and 31 °C; shoot height, shoot dry weight, stem diameter, root penetration, root volume, and root dry weight all reached maxima at 19 °C.[100]
However, whereas strong positive relationships between soil temperature (5 °C to 25 °C) and growth have been found in trembling aspen and balsam poplar, white and other spruce species have shown little or no changes in growth with increasing soil temperature.[99][101][102][103][104] Such insensitivity to soil low temperature may be common among a number of western and boreal conifers.[105]
Soil temperatures are increasing worldwide under the influence of present-day global climate warming, with opposing views about expected effects on carbon capture and storage and feedback loops to climate change[106] Most threats are about permafrost thawing and attended effects on carbon destocking[107] and ecosystem collapse.[108]
Colour
Soil colour is often the first impression one has when viewing soil. Striking colours and contrasting patterns are especially noticeable. The Red River of the South carries sediment eroded from extensive reddish soils like Port Silt Loam in Oklahoma. The Yellow River in China carries yellow sediment from eroding loess soils. Mollisols in the Great Plains of North America are darkened and enriched by organic matter. Podsols in boreal forests have highly contrasting layers due to acidity and leaching.
In general, color is determined by the organic matter content, drainage conditions, and degree of oxidation. Soil color, while easily discerned, has little use in predicting soil characteristics.[109] It is of use in distinguishing boundaries of horizons within a soil profile,[110] determining the origin of a soil's parent material,[111] as an indication of wetness and waterlogged conditions,[112] and as a qualitative means of measuring organic,[113] iron oxide[114] and clay contents of soils.[111] Color is recorded in the Munsell color system as for instance 10YR3/4 Dusky Red, with 10YR as hue, 3 as value and 4 as chroma. Munsell color dimensions (hue, value and chroma) can be averaged among samples and treated as quantitative parameters, displaying significant correlations with various soil[115] and vegetation properties.[116]
Soil color is primarily influenced by soil mineralogy. Many soil colours are due to various iron minerals.[114] The development and distribution of colour in a soil profile result from chemical and biological weathering, especially redox reactions.[112] As the primary minerals in soil parent material weather, the elements combine into new and colourful compounds. Iron forms secondary minerals of a yellow or red colour,[117] organic matter decomposes into black and brown humic compounds,[118] and manganese[119] and sulfur[120] can form black mineral deposits. These pigments can produce various colour patterns within a soil. Aerobic conditions produce uniform or gradual colour changes, while reducing environments (anaerobic) result in rapid colour flow with complex, mottled patterns and points of colour concentration.[121]
Resistivity
Soil resistivity is a measure of a soil's ability to retard the conduction of an electric current. The electrical resistivity of soil can affect the rate of corrosion of metallic structures in contact with the soil.[122] Higher moisture content or increased electrolyte concentration can lower resistivity and increase conductivity, thereby increasing the rate of corrosion.[123][124] Soil resistivity values typically range from about 1 to 100000 Ω·m, extreme values being for saline soils and dry soils overlaying cristalline rocks, respectively.[125]
See also
References
- Samouëlian, Anatja; Cousin, Isabelle; Tabbagh, Alain; Bruand, Ary; Richard, Guy (2005). "Electrical resistivity survey in soil science: a review". Soil and Tillage Research. 83 (2): 173–93. CiteSeerX 10.1.1.530.686. doi:10.1016/j.still.2004.10.004. S2CID 53615967. Retrieved 31 July 2022.
Bibliography
- Donahue, Roy Luther; Miller, Raymond W.; Shickluna, John C. (1977). Soils: An Introduction to Soils and Plant Growth. Prentice-Hall. ISBN 978-0-13-821918-5.
- "Arizona Master Gardener". Cooperative Extension, College of Agriculture, University of Arizona. Retrieved 27 May 2013.
- Stefferud, Alfred, ed. (1957). Soil: The Yearbook of Agriculture 1957. United States Department of Agriculture. OCLC 704186906.
- Kellogg. "We Seek; We Learn". In Stefferud (1957).
- Simonson. "What Soils Are". In Stefferud (1957).
- Russell. "Physical Properties". In Stefferud (1957).
- Richards & Richards. "Soil Moisture". In Stefferud (1957).
- Wadleigh. "Growth of Plants". In Stefferud (1957).
- Allaway. "pH, Soil Acidity, and Plant Growth". In Stefferud (1957).
- Coleman & Mehlich. "The Chemistry of Soil pH". In Stefferud (1957).
- Dean. "Plant Nutrition and Soil Fertility". In Stefferud (1957).
- Allison. "Nitrogen and Soil Fertility". In Stefferud (1957).
- Olsen & Fried. "Soil Phosphorus and Fertility". In Stefferud (1957).
- Reitemeier. "Soil Potassium and Fertility". In Stefferud (1957).
- Jordan & Reisenauer. "Sulfur and Soil Fertility". In Stefferud (1957).
- Holmes & Brown. "Iron and Soil Fertility". In Stefferud (1957).
- Seatz & Jurinak. "Zinc and Soil Fertility". In Stefferud (1957).
- Russel. "Boron and Soil Fertility". In Stefferud (1957).
- Reuther. "Copper and Soil Fertility". In Stefferud (1957).
- Sherman. "Manganese and Soil Fertility". In Stefferud (1957).
- Stout & Johnson. "Trace Elements". In Stefferud (1957).
- Broadbent. "Organic Matter". In Stefferud (1957).
- Clark. "Living Organisms in the Soil". In Stefferud (1957).
- Flemming. "Soil Management and Insect Control". In Stefferud (1957).
https://en.wikipedia.org/wiki/Physical_properties_of_soil
In biology, a pair bond is the strong affinity that develops in some species between a mating pair, often leading to the production and rearing of offspring and potentially a lifelong bond. Pair-bonding is a term coined in the 1940s[1] that is frequently used in sociobiology and evolutionary biology circles. The term often implies either a lifelong socially monogamous relationship or a stage of mating interaction in socially monogamous species. It is sometimes used in reference to human relationships.
https://en.wikipedia.org/wiki/Pair_bond
Secret Beach, officially known as Kauapea Beach, is a beach in Kalihiwai and Kīlauea on the north shore of the island of Kauai, Hawaii. The beach is known for its size, seclusion, and beauty.
https://en.wikipedia.org/wiki/Secret_Beach
| Afghan leopard gecko | |
|---|---|

| |
| A female Afghan Leopard Gecko | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Reptilia |
| Order: | Squamata |
| Family: | Eublepharidae |
| Genus: | Eublepharis |
| Species: | |
| Subspecies: | E. m. afghanicus
|
| Trinomial name | |
| Eublepharis macularius afghanicus Börner 1976
| |
The Afghan leopard gecko (Eublepharis macularius afghanicus) is one of the five subspecies of the common leopard gecko, a small to midsized lizard belonging to the family Gekkonidae.[1] This subspecies was first discovered by entomologist Carl Julius Bernhard Börner in 1976. It is much smaller than other leopard gecko subspecies.
https://en.wikipedia.org/wiki/Afghan_leopard_gecko
The black mummy technique
The black mummy technique (5000 to 3000 BCE) involved taking the dead person's body apart, treating it, and reassembling it. The head, arms, and legs were removed from the trunk; the skin was often removed, too. The body was heat-dried, and the flesh and tissue were completely stripped from the bone by using stone tools. Evidence exists that the bones were dried by hot ashes or coal. After reassembly, the body was then covered with a white ash paste, filling the gaps with grass, ashes, soil, animal hair and more. The paste was also used to fill out the person's normal facial features. The person's skin (including facial skin with a wig attachment of short black human hair) was refitted on the body, sometimes in smaller pieces, sometimes in one almost-whole piece. Sea lion skin was sometimes used as well. Then the skin (or, in the case of children, who were often missing their skin layer, the white ash layer) was painted with black manganese giving their color.[2]
https://en.wikipedia.org/wiki/Chinchorro_mummies#The_black_mummy_technique
| White Sands National Park | |
|---|---|
IUCN category V (protected landscape/seascape)[1] | |
 Aerial view of dunefield | |
| Location | Otero County and Doña Ana County, New Mexico, United States |
| Nearest city | Alamogordo, New Mexico[2] |
| Coordinates | 32°46′45″N 106°10′19″WCoordinates: 32°46′45″N 106°10′19″W[2] |
| Area | 145,762 acres (227.753 sq mi; 589.88 km2)[3]+[4] |
| Established | January 18, 1933 (as a national monument)[3] December 20, 2019 (as a national park)[4] |
| Visitors | 782,469[3] (in 2021) |
| Governing body | National Park Service |
| Website | Official website |
White Sands National Monument Historic District | |
Former U.S. National Monument | |
 Visitor center | |
| Built | 1936–38 (original NRHP buildings) |
|---|---|
| Architect | Lyle E. Bennett, et al. |
| Architectural style | Pueblo Revival |
| NRHP reference No. | 88000751[5] |
| NMSRCP No. | 1491 |
| Significant dates | |
| Added to NRHP | June 23, 1988 |
| Designated NMSRCP | September 9, 1988 |
White Sands National Park is an American national park located in the state of New Mexico and completely surrounded by the White Sands Missile Range. The park covers 145,762 acres (227.8 sq mi; 589.9 km2) in the Tularosa Basin, including the southern 41% of a 275 sq mi (710 km2) field of white sand dunes composed of gypsum crystals. This gypsum dunefield is the largest of its kind on Earth,[6] with a depth of about 30 feet (9.1 m), dunes as tall as 60 feet (18 m), and about 4.5 billion short tons (4.1 billion metric tons) of gypsum sand.
Approximately 12,000 years ago, the land within the Tularosa Basin featured large lakes, streams, grasslands, and Ice Age mammals. As the climate warmed, rain and snowmelt dissolved gypsum from the surrounding mountains and carried it into the basin. Further warming and drying caused the lakes to evaporate and form selenite crystals. Strong winds then broke up crystals and transported them eastward. A similar process continues to produce gypsum sand today.[7]
Thousands of species of animal inhabit the park, a large portion of which are invertebrates. Several animal species feature a white or off-white coloration. At least 45 species are endemic, living only in this park, with 40 of them being moth species. The Tularosa Basin has also seen a number of human inhabitants, from Paleo-Indians 12,000 years ago to modern farmers, ranchers, and miners.
White Sands National Park was originally designated White Sands National Monument on January 18, 1933 by President Herbert Hoover; it was redesignated as a national park by Congress and signed into law by President Donald Trump on December 20, 2019. It is the most visited NPS site in New Mexico, with about 600,000 visitors each year.[8] The park features a drive from the visitor center to the heart of the dunes, picnic areas, backcountry campground in the dunefield, marked hiking trails, and sledding on the dunes. Ranger-guided orientation and nature walks occur at various times and months throughout the year.
https://en.wikipedia.org/wiki/White_Sands_National_Park
| Giant sea bass | |
|---|---|

| |
| A giant sea bass at the California Academy of Sciences | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Actinopterygii |
| Order: | Perciformes |
| Family: | Polyprionidae |
| Genus: | Stereolepis |
| Species: | S. gigas
|
| Binomial name | |
| Stereolepis gigas Ayres, 1859
| |
| Synonyms[2] | |
| |
The giant sea bass (Stereolepis gigas) is a fish native to the North Pacific Ocean. Although commonly referred to as a giant sea bass, black sea bass or giant black sea bass, it is actually a wreckfish in the family Polyprionidae rather than in the sea bass family Serranidae.[3]
https://en.wikipedia.org/wiki/Giant_sea_bass
| Gorilla Falls Exploration Trail | |
|---|---|
 Formerly known as Pangani Forest Exploration Trail from August 1998-May 2016 | |
| Disney's Animal Kingdom | |
| Area | Africa |
| Coordinates | 28°21′36.97″N 81°35′31.72″WCoordinates: 28°21′36.97″N 81°35′31.72″W |
| Status | Operating |
| Opening date | April 22, 1998 |
| Ride statistics | |
| Attraction type | Trail |
| Designer | Walt Disney Imagineering |
| Theme | African Rainforest |
| Stations | 2 |
| Animals | Primates |
| Animal Viewing Locations | 4 |
| Observing Stations | 3 |
| Main animal | Western lowland gorilla |
| Other animals | Black and white colobus monkey, meerkat, yellow-backed duiker, okapi, birds, gerenuk |
The Gorilla Falls Exploration Trail (formerly known as Pangani Forest Exploration Trail from August 1998 to May 2016) is a walkway next to Kilimanjaro Safaris at the Disney's Animal Kingdom in the Walt Disney World Resort, Florida, from which visitors can see African animals. It is about three-eighths of a mile in length. There are "research students" positioned at most locations to give information about the animals and answer questions.
The attraction originally opened on April 22, 1998 as Gorilla Falls Exploration Trail, but the name was changed to Pangani Forest Exploration Trail in August 1998.[1] The attraction reverted to its original name on May 27, 2016.[2]
https://en.wikipedia.org/wiki/Gorilla_Falls_Exploration_Trail
Permanent mold casting is a metal casting process that employs reusable molds ("permanent molds"), usually made from metal. The most common process uses gravity to fill the mold, however gas pressure or a vacuum are also used. A variation on the typical gravity casting process, called slush casting, produces hollow castings. Common casting metals are aluminium, magnesium, and copper alloys. Other materials include tin, zinc, and lead alloys and iron and steel are also cast in graphite molds.[1][2]
Typical products are components such as gears, splines, wheels, gear housings, pipe fittings, fuel injection housings, and automotive engine pistons.[1]
https://en.wikipedia.org/wiki/Permanent_mold_casting
| Doctorfish tang | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | A. chirurgus
|
| Binomial name | |
| Acanthurus chirurgus (Bloch, 1787)
| |

| |
| Synonyms | |
| |
Acanthurus chirurgus, commonly called doctorfish or doctorfish tang in English and barbero rayado or cirujano rayado in Spanish, is a tropical marine fish common in the Atlantic Ocean.
https://en.wikipedia.org/wiki/Doctorfish_tang
This is a list of all genera, species and subspecies of the subfamily Viperinae, otherwise referred to as viperines, true vipers, pitless vipers or Old World vipers. It follows the taxonomy of McDiarmid et al. (1999)[1] and ITIS.[2]
- Atheris, Bush vipers
- Atheris acuminata
- Atheris anisolepis
- Atheris barbouri, Uzungwe mountain bush viper
- Atheris broadleyi
- Atheris ceratophora, Horned bush viper
- Atheris chlorechis, Western bush viper
- Atheris desaixi, Mount Kenya bush viper
- Atheris hetfieldi
- Atheris hirsuta, Tai hairy bush viper
- Atheris hispida, Bristly bush viper
- Atheris katangensis, Katanga mountain bush viper
- Atheris mabuensis, Mount Mabu forest viper
- Atheris matildae, Matilda's horned viper
- Atheris mongoensis
- Atheris nitschei, Great Lakes bush viper
- Atheris rungweensis, Rungwe tree viper
- Atheris squamigera, Rough-scaled bush viper
- Atheris subocularis
- Bitis, Puff adders
- Bitis albanica, Albany adder
- Bitis arietans, Common puff adder
- Bitis arietans arietans, Common puff adder
- Bitis arietans somalica, Somali puff adder
- Bitis armata, Southern adder
- Bitis atropos, Mountain adder
- Bitis caudalis, Horned adder
- Bitis cornuta, Many-horned adder
- Bitis gabonica, Gaboon viper (3003 Viper)
- Bitis gabonica, East African gaboon viper
- Bitis harenna
- Bitis heraldica, Angolan adder
- Bitis inornata, Plain mountain adder
- Bitis nasicornis, Rhinoceros viper
- Bitis parviocula, Ethiopian viper
- Bitis peringueyi, Peringuey's desert adder
- Bitis rhinoceros, West African gaboon viper
- Bitis rubida, Red adder
- Bitis schneideri, Namaqua dwarf adder
- Bitis worthingtoni, Kenyan horned viper
- Bitis xeropaga, Desert mountain adder
- Causus, night adders
- Causus bilineatus, lined night adder, two-striped night adder
- Causus defilippii, snouted night adder
- Causus lichtensteinii, forest night adder
- Causus maculatus, forest rhombic night adder, West African night adder
- Causus rasmusseni
- Causus resimus, green night adder
- Causus rhombeatus, rhombic night adder
- Cerastes, Horned vipers
- Cerastes boehmeii Tunesian horned viper
- Cerastes cerastes, Saharan horned viper
- Cerastes gasperettii, Arabian horned viper
- Cerastes vipera, Sahara sand viper
- Daboia
- Daboia mauritanica, Moorish viper
- Daboia palaestinae, Palestine viper
- Daboia russelii, Russell's viper
- Daboia siamensis, Eastern Russell's viper
- Echis, Saw-scaled vipers
- Echis borkini
- Echis carinatus, Saw-scaled viper
- Echis carinatus astolae, Astola saw-scaled viper
- Echis carinatus carinatus, South Indian saw-scaled viper
- Echis carinatus multisquamatus, Central Asian saw-scaled viper
- Echis carinatus sinhaleyus, Sri Lankan saw-scaled viper
- Echis carinatus sochureki, Sochurek's saw-scaled viper
- Echis coloratus, Painted saw-scaled viper
- Echis hughesi, Hughes' saw-scaled viper
- Echis jogeri, Joger's saw-scaled viper
- Echis khosatzkii
- Echis leucogaster, White-bellied saw-scaled viper
- Echis megalocephalus, Cherlin's saw-scaled viper
- Echis ocellatus, West African saw-scaled viper
- Echis omanensis, Oman saw-scaled viper
- Echis pyramidum, Egyptian saw-scaled viper
- Echis pyramidum aliaborri, Red carpet viper
- Echis pyramidum leakeyi, Kenyan carpet viper
- Echis pyramidum pyramidum, Egyptian saw-scaled viper
- Echis romani
- Eristicophis, McMahon's desert viper
- Eristicophis macmahonii, McMahon's desert viper
- Macrovipera, Large Palearctic vipers
- Macrovipera lebetina, Blunt-nosed viper
- Macrovipera lebetina cernovi
- Macrovipera lebetina lebetina, Blunt-nosed viper
- Macrovipera lebetina obtusa, West-Asian blunt-nosed viper
- Macrovipera lebetina transmediterranea
- Macrovipera lebetina turanica, Turan blunt-nosed viper
- Macrovipera razii
- Macrovipera schweizeri, Milos viper
- Montatheris hindii, Montane viper
- Macrovipera lebetina, Blunt-nosed viper
- Montivipera
- Montivipera albizona, Central Turkish mountain viper
- Montivipera bornmuelleri, Lebanon viper (Endangered)
- Montivipera bulgardaghica, Mount Bulgar viper
- Montivipera kuhrangica, Kuhrang mountain viper
- Montivipera latifii, Latifi's viper
- Montivipera raddei, Rock viper
- Montivipera raddei albicornuta, Iranian mountain viper
- Montivipera raddei kurdistanica , Kurdistan viper
- Montivipera wagneri, Ocellated mountain viper
- Montivipera xanthina, Rock viper
- Proatheris, Lowland swamp viper
- Proatheris superciliaris, Lowland swamp viper
- Pseudocerastes
- Pseudocerastes fieldi, Field's horned viper
- Pseudocerastes persicus, Persian horned viper
- Pseudocerastes urarachnoides, Iranian spider viper
- Vipera, Palearctic vipers
- Vipera altaica , Kazachstan viper
- Vipera ammodytes, Sand viper
- Vipera ammodytes ammodytes, Western sand viper
- Vipera ammodytes gregorwallneri
- Vipera ammodytes meridionalis, Eastern sand viper
- Vipera ammodytes montandoni, Transdanubian sand viper
- Vipera anatolica , Anatolian viper (critically endangered)
- Vipera aspis, Asp viper
- Vipera aspis aspis, European asp
- Vipera aspis atra, Black asp
- Vipera aspis francisciredi, Central Italian asp
- Vipera aspis hugyi, Southern Italian asp
- Vipera aspis zinnikeri, Gascony asp
- Vipera barani, Baran's adder
- Vipera berus, common viper, adder
- Vipera berus berus, Common European adder
- Vipera berus bosniensis, Balkan cross adder
- Vipera berus sachalinensis, Sakhalin Island adder
- Vipera darevskii, Darevsky's viper
- Vipera dinniki, Dinnik's viper
- Vipera ebneri , Iranian Mountain steppe viper
- Vipera eriwanensis, Armenian steppe viper
- Vipera graeca, Greek meadow viper
- Vipera kaznakovi, Caucasus viper
- Vipera latastei, Lataste's viper
- Vipera latastei gaditana
- Vipera latastei latastei, Lataste's viper
- Vipera lotievi, Caucasian meadow viper
- Vipera magnifica, Magnificent viper
- Vipera monticola, Atlas mountain viper
- Vipera nikolskii, Nikolski's viper
- Vipera olguni
- Vipera orlovi, Orlov's viper (critically Endangered)
- Vipera pontica, Pontic adder, Black sea viper
- Vipera renardi, Steppe viper
- Vipera renardi tienshanica, Kasackstan steppe viper
- Vipera seoanei, Baskian viper
- Vipera seoanei cantabrica
- Vipera seoanei seoanei, Baskian viper
- Vipera shemakhensis
- Vipera transcaucasiana, Transcaucasian longnose viper
- Vipera ursinii, Meadow vipers
- Vipera ursinii macrops, Albanian meadow viper
- Vipera ursinii moldavica, Romanian meadow viper
- Vipera ursinii rakosiensis, Hungarian meadow viper
- Vipera ursinii wettsteini, Provence meadow viper
- Vipera ursinii ursini , Italian meadow viper
- Vipera walser, Piemont viper
References
- "Viperinae". Integrated Taxonomic Information System. Retrieved 23 March 2007.
https://en.wikipedia.org/wiki/List_of_viperine_species_and_subspecies
| Sea of Azov | |
|---|---|
 Sea of Azov shoreline at Yalta, Donetsk Oblast | |
 Sea of Azov, upper right | |
| Coordinates | 46°N 37°ECoordinates: 46°N 37°E |
| Type | Sea |
| Primary inflows | Don and Kuban |
| Basin countries | Russia, Ukraine |
| Max. length | 360 km (220 mi)[1] |
| Max. width | 180 km (110 mi)[1] |
| Surface area | 39,000 km2 (15,000 sq mi)[1][2] |
| Average depth | 7 metres (23 ft)[1] |
| Max. depth | 14 m (46 ft)[1] |
| Water volume | 290 km3 (240×106 acre⋅ft)[1] |
The Sea of Azov (Crimean Tatar: Azaq deñizi; Russian: Азовское море, romanized: Azovskoye more; Ukrainian: Азовське море, romanized: Azovs'ke more)[3] is an inland shelf sea in Eastern Europe connected to the Black Sea by the narrow (about 4 km or 2.5 mi) Strait of Kerch, and is sometimes regarded as a northern extension of the Black Sea.[4][5] The sea is bounded by Russia on the east, and by Ukraine on the northwest and southwest, currently under Russian occupation. It is an important access route for Central Asia, from the Caspian Sea via the Volga-Don Canal.
The sea is largely affected by the inflow of the Don, Kuban, and other rivers, which bring sand, silt, and shells, which in turn form numerous bays, limans, and narrow spits. Because of these deposits, the sea bottom is relatively smooth and flat with the depth gradually increasing toward the middle. Because of the river inflow, water in the sea has low salinity and a high amount of biomass (such as green algae) that affects the water colour. Abundant plankton result in unusually high fish productivity. The sea shores and spits are low; they are rich in vegetation and bird colonies. The Sea of Azov is the shallowest sea in the world, with the depth varying between 0.9 and 14 metres (3 and 46 ft).[1][6][7][8][9] There is a constant outflow of water from the Sea of Azov to the Black Sea.
https://en.wikipedia.org/wiki/Sea_of_Azov
| Gabara subnivosella | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Lepidoptera |
| Superfamily: | Noctuoidea |
| Family: | Erebidae |
| Genus: | Gabara |
| Species: | G. subnivosella
|
| Binomial name | |
| Gabara subnivosella Walker, 1866
| |
| Synonyms | |
| |
Gabara subnivosella, the wet sand savannah moth, is a moth in the family Erebidae. The species was first described by Francis Walker in 1866. It is found in North America from Manitoba south to Maryland, Massachusetts and New York.
The wingspan is about 25 mm.
Adults are extremely variable in colour and maculation (spots). The colour ranges from nearly white to dark grey and immaculate to specimens with a central nearly black streak across the width of the forewing.
https://en.wikipedia.org/wiki/Gabara_subnivosella
Demersal fish, also known as groundfish, live and feed on or near the bottom of seas or lakes (the demersal zone).[1] They occupy the sea floors and lake beds, which usually consist of mud, sand, gravel or rocks.[1] In coastal waters they are found on or near the continental shelf, and in deep waters they are found on or near the continental slope or along the continental rise. They are not generally found in the deepest waters, such as abyssal depths or on the abyssal plain, but they can be found around seamounts and islands. The word demersal comes from the Latin demergere, which means to sink.
Demersal fish are bottom feeders. They can be contrasted with pelagic fish which live and feed away from the bottom in the open water column. Demersal fish fillets contain little fish oil (one to four percent), whereas pelagic fish can contain up to 30 percent.[not verified in body]
https://en.wikipedia.org/wiki/Demersal_fish
| Florida sand darter | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Actinopterygii |
| Order: | Perciformes |
| Family: | Percidae |
| Genus: | Ammocrypta |
| Species: | A. bifascia
|
| Binomial name | |
| Ammocrypta bifascia J. D. Williams, 1975
| |
| Synonyms[2] | |
| |
The Florida sand darter ('Ammocrypta bifascia) is a species of freshwater ray-finned fish, a darter from the subfamily Etheostomatinae, part of the family Percidae, which also contains the perches, ruffes and pikeperches. It is endemic to Gulf Coast drainages from the Aplalachicola to the Perdido River in Florida and southern Alabama.[2] It inhabits streams with waters that are clear to tannin-stained where there are shifting sand bottoms and a moderate to fast flow. It is most frequently encountered where there is a moderate current in medium-sized to large streams, but it will enter smaller streams on occasion.[1] Its appearance is identical to the naked sand darter aside from 2 black bands on each dorsal fin. This species can reach a length of 7.1 cm (2.8 in), though most are only about 4.7 cm (1.9 in) in length, at depths of 61 to 122 centimetres (2.00 to 4.00 ft).[2] The Florida sand darter was first formally described in 1975 by James D. Williams with the type locality given as the Choctawhatchee River, 2.4 kilometres (1.5 mi) west of Pittman, Florida.[3] This species forms a clade with the naked sand darter (A. beanii) the Western sand darter (A. clara).[4]
https://en.wikipedia.org/wiki/Florida_sand_darter
The Slavic creation myth is a cosmogonic myth in Slavic mythology that explains how the world was created, who created it, and what principles guide it. This myth, in its Christianized form, survived until the nineteenth and twentieth century in various parts of the Slavdom in chronicles or folklore. In the Slavic mythology there are three versions of this myth: the first version is the so-called earth-diver myth, which intertwines two main motifs: the dualistic motif – the cooperation of God and the Devil (that is, the "good god" and the "bad god") is required to create the world, and the oceanic motif – the pre-existence water, where the seed of the Earth comes from; the second version speaks about the origin of the universe and the world from the Cosmic Egg and the World Tree; the third one about creation from a dismemberment of a primordial being.
https://en.wikipedia.org/wiki/Slavic_creation_myth
Holothuria edulis
| Holothuria edulis | |
|---|---|

| |
| Pink form from Malaysia | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Echinodermata |
| Class: | Holothuroidea |
| Order: | Holothuriida |
| Family: | Holothuriidae |
| Genus: | Holothuria |
| Subgenus: | Halodeima |
| Species: | H. edulis
|
| Binomial name | |
| Holothuria edulis | |
| Synonyms[2] | |
| |
Holothuria edulis, commonly known as the edible sea cucumber or the pink and black sea cucumber, is a species of echinoderm in the family Holothuriidae. It was placed in the subgenus Halodeima by Pearson in 1914, making its full scientific name Holothuria (Halodeima) edulis.[2] It is found in shallow water in the tropical Indo-Pacific Ocean.[1]
Description
Holothuria edulis is a medium-sized sea cucumber reaching a length of about 30 centimetres (12 in). It has a roughly cylindrical shape with rounded ends but can retract and expand its body and adopt different shapes. It is usually soft and pliable with a smooth skin but, due to the special characteristics of its connective tissue, it can become firm and rigid. The body is lined with longitudinal rows of small tube feet which can be withdrawn into the body wall, leaving small hollows. About twenty tube feet in a ring round the mouth are modified into feeding tentacles. This sea cucumber is usually a dark reddish-black colour on its upper side and a pinkish-mauve colour below, but can be grey or dark brown.[3][4][5]
Distribution and habitat
Holothuria edulis is a common and widespread species in the Indo-Pacific Ocean. It lives on the seabed at depths down to 20 metres (66 ft). Its range extends from the Red Sea and East African coast to Sri Lanka, Japan, China, Indonesia, the Philippines, northern Australia and various Pacific islands.[1] It is found in a number of different habitats including on sandy or muddy substrates, on coral rubble and in seagrass meadows. It can be found on inner and outer reef flats, on back reef slopes and in lagoons.[2]
Biology
Holothuria edulis is mainly nocturnal and tends to hide during the day under rocks or pieces of coral. It is a detritivore and feeds by ingesting sand and debris which has accumulated on the seabed which it picks up using its feeding tentacles. Sand is pushed into the mouth and any organic matter present, including the biofilm round the grains, is digested as the sand passes through the gut. The indigestible matter is expelled from the anus leaving a sand ridge as the animal moves around. During its feeding activities, the sea cucumber churns up the top few centimetres of seabed and aerates the sediment.[4]
Locomotion in Holothuria edulis is very slow. It moves mainly by peristaltic action of its body wall, assisted to a limited extent by its tube feet. It can also anchor its feeding tentacles into the sand and haul itself along. If it gets overturned, it can use its feeding tentacles to help right itself.[4]
Holothuria edulis has separate sexes and spawns at any time of year with gametes being liberated into the water column. The larvae are planktonic. This sea cucumber can also reproduce asexually by breaking into two parts, each of which then regrows the missing organs.[4]
Human use
As food
As its name implies, Holothuria edulis is edible. It is dried and sold as "bêche-de-mer" or "trepang" in China and Indonesia.[6]
As aquarium animal
Holothuria edulis is common in the aquarium trade for its colors and its ability to eat detritus. It needs a tank size of 50 US gallons (190 litres) or more. It is easy to care for but may release a toxin when stressed, although this only happens very rarely.[7]
References
- Sea Cucumber. Sea Cucumber. LiveAquaria. Accessed August 30, 2016
https://en.wikipedia.org/wiki/Holothuria_edulis
| Mottled sand grasshopper | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | S. collare
|
| Binomial name | |
| Spharagemon collare (Scudder, 1872)
| |
Spharagemon collare, the mottled sand grasshopper, is found in sandy-soiled, grassy areas of northern United States and southern Canada. They are known to be a minor pest of wheat crops; however, populations are rarely large enough to cause appreciable damage.[1][2]
https://en.wikipedia.org/wiki/Spharagemon_collare
| Chinese mountain cat | |
|---|---|

| |
| Chinese mountain cat in Xining Zoo | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Carnivora |
| Suborder: | Feliformia |
| Family: | Felidae |
| Subfamily: | Felinae |
| Genus: | Felis |
| Species: | |
| Binomial name | |
| Felis bieti[1][2] Milne-Edwards, 1892
| |

| |
| Distribution of the Chinese mountain cat, 2022[3] | |
The Chinese mountain cat (Felis bieti), also known as Chinese desert cat and Chinese steppe cat, is a small wild cat endemic to western China that has been listed as Vulnerable on the IUCN Red List since 2002, as the effective population size may be fewer than 10,000 mature breeding individuals.[3]
It was provisionally classified as a wildcat subspecies with the name F. silvestris bieti in 2007.[4] It is recognised as a valid species since 2017, as it is morphologically distinct from wildcats.[1]
https://en.wikipedia.org/wiki/Chinese_mountain_cat
| Common tern | |
|---|---|

| |
| 1:21 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Aves |
| Order: | Charadriiformes |
| Family: | Laridae |
| Genus: | Sterna |
| Species: | S. hirundo
|
| Binomial name | |
| Sterna hirundo | |

| |
Breeding Resident Non-breeding Passage Vagrant (seasonality uncertain)
| |
| Synonyms | |
| |
The common tern[2] (Sterna hirundo) is a seabird in the family Laridae. This bird has a circumpolar distribution, its four subspecies breeding in temperate and subarctic regions of Europe, Asia and North America. It is strongly migratory, wintering in coastal tropical and subtropical regions. Breeding adults have light grey upperparts, white to very light grey underparts, a black cap, orange-red legs, and a narrow pointed bill. Depending on the subspecies, the bill may be mostly red with a black tip or all black. There are several similar species, including the partly sympatric Arctic tern, which can be separated on plumage details, leg and bill colour, or vocalisations.
Breeding in a wider range of habitats than any of its relatives, the common tern nests on any flat, poorly vegetated surface close to water, including beaches and islands, and it readily adapts to artificial substrates such as floating rafts. The nest may be a bare scrape in sand or gravel, but it is often lined or edged with whatever debris is available. Up to three eggs may be laid, their dull colours and blotchy patterns providing camouflage on the open beach. Incubation is by both sexes, and the eggs hatch in around 21–22 days, longer if the colony is disturbed by predators. The downy chicks fledge in 22–28 days. Like most terns, this species feeds by plunge-diving for fish, either in the sea or in freshwater, but molluscs, crustaceans and other invertebrate prey may form a significant part of the diet in some areas.
Eggs and young are vulnerable to predation by mammals such as rats and American mink, and large birds including gulls, owls and herons. Common terns may be infected by lice, parasitic worms, and mites, although blood parasites appear to be rare. Its large population and huge breeding range mean that this species is classed as being of least concern, although numbers in North America have declined sharply in recent decades. Despite international legislation protecting the common tern, in some areas, populations are threatened by habitat loss, pollution, or the disturbance of breeding colonies.
https://en.wikipedia.org/wiki/Common_tern
| Herons Temporal range: Early Oligocene–Holocene
| |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Aves |
| Order: | Pelecaniformes |
| Suborder: | Ardei |
| Family: | Ardeidae Leach, 1820 |
| Type genus | |
| Ardea Linnaeus, 1758 | |
| Genera | |
|
18 extant, see text | |

| |
| Global distribution of herons | |
| Synonyms | |
|
Cochlearidae | |
The herons are long-legged, long-necked, freshwater and coastal birds in the family Ardeidae,[2] with 72 recognised species, some of which are referred to as egrets or bitterns rather than herons. Members of the genera Botaurus and Ixobrychus are referred to as bitterns, and, together with the zigzag heron, or zigzag bittern, in the monotypic genus Zebrilus, form a monophyletic group within the Ardeidae. Egrets do not form a biologically distinct group from herons, and tend to be named differently because they are mainly white or have decorative plumes in breeding plumage. Herons, by evolutionary adaptation, have long beaks.
The classification of the individual heron/egret species is fraught with difficulty, and no clear consensus exists about the correct placement of many species into either of the two major genera, Ardea and Egretta. Similarly, the relationships of the genera in the family are not completely resolved. However, one species formerly considered to constitute a separate monotypic family, the Cochlearidae or the boat-billed heron, is now regarded as a member of the Ardeidae.
Although herons resemble birds in some other families, such as the storks, ibises, spoonbills, and cranes, they differ from these in flying with their necks retracted, not outstretched. They are also one of the bird groups that have powder down. Some members of this group nest colonially in trees, while others, notably the bitterns, use reed beds. A group of them is called a "siege."[3]
https://en.wikipedia.org/wiki/Heron
| Gull (commonly seagull) Temporal range: Early Oligocene – Present
| |
|---|---|

| |
| Adult Vega gull | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Aves |
| Order: | Charadriiformes |
| Suborder: | Lari |
| Family: | Laridae |
| Genera | |
|
11, see text | |
Gulls, or colloquially seagulls, are seabirds of the family Laridae in the suborder Lari. They are most closely related to the terns and skimmers and only distantly related to auks, and even more distantly to waders. Until the 21st century, most gulls were placed in the genus Larus, but that arrangement is now considered polyphyletic, leading to the resurrection of several genera.[1] An older name for gulls is mews, which is cognate with German Möwe, Danish måge, Swedish mås, Dutch meeuw, Norwegian måke/måse and French mouette, and can still be found in certain regional dialects.[2][3][4]
https://en.wikipedia.org/wiki/Gull
| Owl Temporal range: Late Paleocene to recent
| |
|---|---|

| |
| Left Strigidae: Tawny owl (Strix aluco), Eurasian eagle-owl (Bubo bubo), Little owl (Athene noctua), Northern saw-whet owl (Aegolius acadicus); Right Tytonidae: Barn owl (Tyto alba), Lesser sooty owl (Tyto multipunctata), Tasmanian masked owl (Tyto novaehollandiae castanops), Sri Lanka bay owl (Phodilus assimilis). | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Aves |
| Clade: | Telluraves |
| Order: | Strigiformes Wagler, 1830 |
| Families | |
|
Strigidae | |

| |
| Range of the owl, all species. | |
| Synonyms | |
|
Strigidae sensu Sibley & Ahlquist | |
Owls are birds from the order Strigiformes[1] (/ˈstrɪdʒəfɔːrmiːz/), which includes over 200 species of mostly solitary and nocturnal birds of prey typified by an upright stance, a large, broad head, binocular vision, binaural hearing, sharp talons, and feathers adapted for silent flight. Exceptions include the diurnal northern hawk-owl and the gregarious burrowing owl.
Owls hunt mostly small mammals, insects, and other birds, although a few species specialize in hunting fish. They are found in all regions of the Earth except the polar ice caps and some remote islands.
Owls are divided into two families: the true (or typical) owl family, Strigidae, and the barn-owl family, Tytonidae.[2]
A group of owls is called a "parliament".[3]
https://en.wikipedia.org/wiki/Owl
| Cariamiformes Temporal range:
Suspected, but still not confirmed, late Cretaceous origin by molecular clock | |
|---|---|

| |
| Red-legged seriema, Cariama cristata (Cariamidae) | |

| |
| Kelenken (Phorusrhacidae) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Aves |
| Clade: | Australaves |
| Order: | Cariamiformes Fürbringer, 1888 |
| Families | |
|
Cariamidae | |
Cariamiformes (or Cariamae) is an order of primarily flightless birds that has existed for over 60 million years. The group includes the family Cariamidae (seriemas) and the extinct families Phorusrhacidae, Bathornithidae, Idiornithidae and Ameghinornithidae. Though traditionally considered a suborder within Gruiformes, both morphological and genetic studies[3] show that it belongs to a separate group of birds, Australaves, whose other living members are Falconidae, Psittaciformes and Passeriformes.[4]
https://en.wikipedia.org/wiki/Cariamiformes
Wieslochia fossil
https://en.wikipedia.org/wiki/Passerine
| Cathartiformes Temporal range: Eocene to present
| |
|---|---|

| |
| California condor | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Aves |
| Clade: | Accipitrimorphae |
| Order: | Cathartiformes Coues, 1884 |
| Subtaxa | |
The order Cathartiformes /kəˈθɑːrtɪfɔːrmiːz/ of raptors or birds of prey includes the New World vultures and the now-extinct Teratornithidae.[1] These raptors are classified by most taxonomic authorities in the order Accipitriformes (which includes the eagles and hawks). In the past, they were considered to be a sister group to the storks of the order Ciconiiformes based on DNA–DNA hybridization and morphology.[2][3] However, a 2021 analysis of mitochondrial genes among Accipitrimorphae, which include Cathartiformes, reinforced prior findings on the phylogenetic relationships between Cathartiformes and other subfamilies of Accipitriformes.[4]
https://en.wikipedia.org/wiki/Cathartiformes
| Phalangeriformes Temporal range:
| |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Infraclass: | Marsupialia |
| Order: | Diprotodontia |
| Suborder: | Phalangeriformes Szalay in Archer, 1982 |
| Groups included | |
| Cladistically included but traditionally excluded taxa | |
Phalangeriformes /fəˈlændʒərɪfɔːrmiːz/ is a paraphyletic[1] suborder of about 70 species of small to medium-sized arboreal marsupials native to Australia, New Guinea, and Sulawesi.[2] The species are commonly known as possums, gliders, and cuscus. The common name "possum" for various Phalangeriformes species derives from the creatures' resemblance to the opossums of the Americas (the term comes from Powhatan language aposoum "white animal", from Proto-Algonquian *wa·p-aʔɬemwa "white dog"). However, although opossums are also marsupials, Australasian possums are more closely related to other Australasian marsupials such as kangaroos.
Phalangeriformes are quadrupedal diprotodont marsupials with long tails. The smallest species, indeed the smallest diprotodont marsupial, is the Tasmanian pygmy possum, with an adult head-body length of 70 mm (2+3⁄4 in) and a weight of 10 g (3⁄8 oz). The largest are the two species of bear cuscus, which may exceed 7 kg (15 lb 7 oz). Phalangeriformes species are typically nocturnal and at least partially arboreal. They inhabit most vegetated habitats, and several species have adjusted well to urban settings. Diets range from generalist herbivores or omnivores (the common brushtail possum) to specialist browsers of eucalyptus (greater glider), insectivores (mountain pygmy possum) and nectar-feeders (honey possum).
https://en.wikipedia.org/wiki/Phalangeriformes
Evolution and systematics
Recent phylogenetic studies place owls within the clade Telluraves, most closely related to the Accipitrimorphae and the Coraciimorphae,[35][36] although the exact placement within Telluraves is disputed.[37][38]
See below cladogram:
| Telluraves |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
Cladogram of Telluraves relationships based on Braun & Kimball (2021)[39]
Some 220 to 225 extant species of owls are known, subdivided into two families: 1. true owls or typical owls family (Strigidae) and 2. barn-owls family (Tytonidae). Some entirely extinct families have also been erected based on fossil remains; these differ much from modern owls in being less specialized or specialized in a very different way (such as the terrestrial Sophiornithidae). The Paleocene genera Berruornis and Ogygoptynx show that owls were already present as a distinct lineage some 60–57 million years ago (Mya), hence, possibly also some 5 million years earlier, at the extinction of the non-avian dinosaurs. This makes them one of the oldest known groups of non-Galloanserae landbirds. The supposed "Cretaceous owls" Bradycneme and Heptasteornis are apparently non-avialan maniraptors.[40]
https://en.wikipedia.org/wiki/Owl
| Karakum (Desert) | |
|---|---|
 Sand dunes in the desert, in Turkmenistan | |
 The Karakum Desert by NASA World Wind | |
| Area | 350,000 km2 (140,000 sq mi) |
| Geography | |
| Country | Turkmenistan |
| State/Province | Central Asia |
| Coordinates | 40°30′N 60°00′ECoordinates: 40°30′N 60°00′E |
The Karakum Desert, also spelled Kara-Kum and Gara-Gum (Turkmen: Garagum, pronounced [ɡɑɾɑˈɡʊm]; Russian: Караку́мы, tr. Karakumy, IPA: [kərɐˈkumɨ]), is a desert in Central Asia. Its name in Turkic languages means "black sand": "kum" means sand; "kara" is a contraction of garaňky: "dark" or may pre-date that (be a derivation from a likely broader meaning which the word for black bore: gara) in this language family.[a] This refers to the shale-rich sand generally beneath the sand of much of the desert.[1] It occupies about 70 percent, 350,000 km2 (140,000 sq mi), of Turkmenistan.
The population is sparse, with an average of one person per 6.5 km2 (2.5 sq mi). Rainfall is also rare, ranging from 70 to 150 mm (3 to 6 in) per year.[2]
https://en.wikipedia.org/wiki/Karakum_Desert
| Sand Hollow State Park | |
|---|---|
IUCN category V (protected landscape/seascape) | |
 Red Mountain in Sand Hollow State Park | |
| Location | Washington County, Utah, United States |
| Coordinates | 37°6′56″N 113°22′35″WCoordinates: 37°6′56″N 113°22′35″W |
| Area | 20,611 acres (83.41 km2)[1] |
| Elevation | 3,000 ft (910 m)[2] |
| Established | 2003[2] |
| Visitors | 183691 (in 2011)[3] |
| Operator | Utah State Parks |
Sand Hollow State Park is a state park located in Utah, USA, featuring a 1,322-acre (535 ha) reservoir and an extensive off highway vehicle recreation area on Sand Mountain. The park is near the town of Hurricane.
The park was officially dedicated in April 2003 and surrounds the Sand Hollow Reservoir. Sand Hollow quickly became a popular site for camping, fishing, boating, and ATV riding on nearby sand dunes.
https://en.wikipedia.org/wiki/Sand_Hollow_State_Park
| Sand cat | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Carnivora |
| Suborder: | Feliformia |
| Family: | Felidae |
| Subfamily: | Felinae |
| Genus: | Felis |
| Species: | F. margarita[1]
|
| Binomial name | |
| Felis margarita[1] Loche, 1858
| |
| Subspecies | |
|
F. m. margarita Loche, 1858 | |

| |
| Distribution of the sand cat in 2016[2] | |
| Synonyms[1] | |
|
| |
List |
The sand cat (Felis margarita) is a small wild cat that inhabits sandy and stony deserts far from water sources. With its sandy to light grey fur, it is well camouflaged in a desert environment. Its head-and-body length ranges from 39–52 cm (15–20 in) with a 23–31 cm (9.1–12.2 in) long tail. Its 5–7 cm (2.0–2.8 in) short ears are set low on the sides of the head, aiding detection of prey moving underground. The long hair covering the soles of its paws insulates its pads against the extremely hot and cold temperatures in deserts.
https://en.wikipedia.org/wiki/Sand_cat
Black Mountain is a summit in the Cleveland National Forest of the Peninsular Ranges in eastern San Diego County, California, north of Ramona. The peak is measured at 4,048 feet (1,234 m), and is sometimes referenced as Big Black Mountain to distinguish it from the smaller Rancho Peñasquitos Black Mountain Open Space Park in the city of San Diego. Black Mountain offers a WX channel for people who wish to tune in on radios to hear the weather; channel is located on VHF frequency 162.4000 MHz.
Black Mountain is home to one of the largest remaining tracts of the threatened Engelmann Oak (Quercus engelmannii).[4][5]
https://en.wikipedia.org/wiki/Black_Mountain_(San_Diego_County,_California)
Punaluʻu Beach (also called Black Sand Beach) is a beach between Pāhala and Nāʻālehu on the Big Island of the U.S. state of Hawaii. The beach has black sand made of basalt and created by lava flowing into the ocean which explodes as it reaches the ocean and cools. This volcanic activity is in the Hawaiʻi Volcanoes National Park. Punaluʻu is frequented by endangered hawksbill and green turtles, which can often be seen basking on the black sand.
https://en.wikipedia.org/wiki/Punalu%CA%BBu_Beach
The Beach
The swimming area is very rocky, and it can be dangerous to swim. The beach also has a large amount of underground fresh water that flows in it. This fresh water is very cold and looks almost like gasoline mixing with the water. Legend has it that in the time of drought, the ancient Hawaiians living in the area would dive underwater with a jug to get their fresh water. In the Hawaiian language puna luʻu means "spring [water] diver for".[1] The beach is located at coordinates 19.136°N 155.504°WCoordinates: 19.136°N 155.504°W. Access is from the Hawaii Belt Road: take Ninole loop road or the entrance to the Sea Mountain Resort. Camping is permitted at the Punaluʻu Black Sand Beach Park.[2]
https://en.wikipedia.org/wiki/Punalu%CA%BBu_Beach
Black Diamond Mines | |
 | |
| Nearest city | Antioch, California |
|---|---|
| Coordinates | 37°57′1″N 121°51′25″WCoordinates: 37°57′1″N 121°51′25″W |
| NRHP reference No. | 91001425[1] |
| CHISL No. | 932[2] |
| Added to NRHP | October 02, 1991 |
The Black Diamond Mines Regional Preserve is a 6,000-acre (2,400 ha) park located north of Mount Diablo in Contra Costa County, California under the administration of the East Bay Regional Park District (EBRPD). The district acquired the property in 1973. The preserve contains relics of 3 mining towns, former coal and sand mines, and offers guided tours of a former sand mine. The 60 miles (97 km) of trails in the Preserve cross rolling foothill terrain covered with grassland, California oak woodland, California mixed evergreen forest, and chaparral.
https://en.wikipedia.org/wiki/Black_Diamond_Mines_Regional_Preserve
| Teton Range | |
|---|---|
 Teton Range, from the Snake River overlook, by Ansel Adams | |
| Highest point | |
| Peak | Grand Teton |
| Elevation | 13,775 ft (4,199 m) NAVD 88[1] |
| Coordinates | 43°44′28″N 110°48′06″W |
| Dimensions | |
| Length | 40 mi (64 km) |
| Width | 12 mi (19 km) |
| Geography | |
| Country | United States |
| State | Wyoming |
| Range coordinates | 43°45′N 110°50′WCoordinates: 43°45′N 110°50′W |
| Parent range | Rocky Mountains |
The Teton Range is a mountain range of the Rocky Mountains in North America. It extends for approximately 40 miles (64 km) in a north–south direction through the U.S. state of Wyoming, east of the Idaho state line. It is south of Yellowstone National Park, and most of the east side of the range is within Grand Teton National Park.
One theory says the early French voyageurs named the range les trois tétons ("the three nipples") after the breast-like shapes of its peaks.[2] Another theory says the range is named for the Teton Sioux (from Thítȟuŋwaŋ), also known as the Lakota people.[3] It is likely that the local Shoshone people once called the whole range Teewinot, meaning "many pinnacles".[4]
The principal summits of the central massif, sometimes referred to as the Cathedral Group, are Grand Teton (13,775 feet (4,199 m)), Mount Owen (12,928 feet (3,940 m)), Teewinot (12,325 feet (3,757 m)), Middle Teton (12,804 feet (3,903 m)) and South Teton (12,514 feet (3,814 m)). Other peaks in the range include Mount Moran (12,605 feet (3,842 m)), Mount Wister (11,490 feet (3,500 m)), Buck Mountain (11,938 feet (3,639 m)) and Static Peak (11,303 feet (3,445 m)).
https://en.wikipedia.org/wiki/Teton_Range
The following sortable table comprises the 477 mountain peaks of the United States with at least 3,000 m (9,843 ft) of topographic elevation and at least 500 m (1,640 ft) of topographic prominence.[1]
The summit of a mountain or hill may be measured in three principal ways:
- The topographic elevation of a summit measures the height of the summit above a geodetic sea level.[2][3]
- The topographic prominence of a summit is a measure of how high the summit rises above its surroundings.[4][3]
- The topographic isolation (or radius of dominance) of a summit measures how far the summit lies from its nearest point of equal elevation.[5]
In the United States, only Denali exceeds 6000 meters (19,685 feet) elevation. Four major summits exceed 5000 meters (16,404 feet), nine exceed 4500 meters (14,764 feet), 104 exceed 4000 meters (13,123 feet), 246 exceed 3500 meters (11,483 feet), and the following 477 major summits exceed 3000 meters (9843 feet) elevation.
https://en.wikipedia.org/wiki/List_of_the_highest_major_summits_of_the_United_States
| American black bear | |
|---|---|

| |
| An American black bear in Manitoba's Riding Mountain National Park | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Carnivora |
| Family: | Ursidae |
| Genus: | Ursus |
| Species: | U. americanus
|
| Binomial name | |
| Ursus americanus Pallas, 1780
| |
| Subspecies | |
|
16, see text | |

| |
| American black bear range[1]
Present-day range
| |
| Synonyms | |
|
Euarctos americanus | |
The American black bear (Ursus americanus), also known as the black bear or sometimes in European languages baribal,[3] is a species of medium-sized bear endemic to North America. It is the continent's smallest and most widely distributed bear species. The American black bear is an omnivore, with its diet varying greatly depending on season and location. It typically lives in largely forested areas, but will leave forests in search of food, and is sometimes attracted to human communities due to the immediate availability of food.
The International Union for Conservation of Nature (IUCN) lists the American black bear as a least-concern species, due to its widespread distribution and a large population estimated to be twice that of all other bear species combined. Along with the brown bear (Ursus arctos), it is one of only two modern bear species not considered by the IUCN to be globally threatened with extinction.
https://en.wikipedia.org/wiki/American_black_bear
| Leonberger | |||||
|---|---|---|---|---|---|
 | |||||
| Common nicknames | Leo | ||||
| Origin | Germany | ||||
| |||||||||||||||||||
| |||||||||||||
| Dog (domestic dog) | |||||||||||||
The Leonberger is a dog breed, whose name derives from the city of Leonberg in Baden-Württemberg, Germany.
https://en.wikipedia.org/wiki/Leonberger
Ironsand, also known as iron-sand or iron sand, is a type of sand with heavy concentrations of iron. It is typically dark grey or blackish in colour.
It is composed mainly of magnetite, Fe3O4, and also contains small amounts of titanium, silica, manganese, calcium and vanadium.[1]
Ironsand has a tendency to heat up in direct sunlight, causing temperatures high enough to cause minor burns. As such it forms a hazard in New Zealand at popular west-coast surf beaches such as Piha.[2]
https://en.wikipedia.org/wiki/Ironsand
 Earliest known edition of the book from the 16th century | |
| Author | Wu Cheng'en |
|---|---|
| Original title | 西遊記 |
| Country | Ming China |
| Language | Chinese |
| Genre | Gods and demons fiction, Chinese mythology, fantasy, adventure |
| Set in | China, 7th century AD |
Publication date | c. 1592 (print)[1] |
Published in English | 1942 (abridged) 1977–1983 (complete) |
| 895.1346 | |
Original text | 西遊記 at Chinese Wikisource |
| Journey to the West | |||
|---|---|---|---|
 Journey to the West in Traditional (top) and Simplified (bottom) Chinese characters | |||
| Traditional Chinese | 西遊記 | ||
| Simplified Chinese | 西游记 | ||
| Literal meaning | "West Journey Record" | ||
|
Journey to the West (Chinese: 西遊記; pinyin: Xī Yóu Jì; Wade–Giles: Hsi1 Yu2 Chi4) is a Chinese novel published in the 16th century during the Ming dynasty and attributed to Wu Cheng'en. It is regarded as one of the greatest Classic Chinese Novels, and has been described as arguably the most popular literary work in East Asia.[2] Arthur Waley's 1942 abridged translation, Monkey, is known in English-speaking countries.
The novel is an extended account of the legendary pilgrimage of the Tang dynasty Buddhist monk Xuanzang, who traveled to the "Western Regions" (Central Asia and India) to obtain Buddhist sūtras (sacred texts) and returned after many trials and much suffering. The monk is referred to as Tang Sanzang in the novel. The novel retains the broad outline of Xuanzang's own account, Great Tang Records on the Western Regions, but adds elements from folk tales and the author's invention: Gautama Buddha gives this task to the monk and provides him with three protectors who agree to help him as an atonement for their sins. These disciples are Sun Wukong, Zhu Bajie, and Sha Wujing, together with a dragon prince who acts as Tang Sanzang's steed, a white horse. The group of pilgrims journeys towards enlightenment by the power and virtue of cooperation.
Journey to the West has strong roots in Chinese folk religion, Chinese mythology, Confucianism, Taoist, and Buddhist theology, and the pantheon of Taoist immortals and Buddhist bodhisattvas are still reflective of some Chinese religious attitudes today. Enduringly popular,[3] the novel is at once a comic adventure story, a humorous satire of Chinese bureaucracy, a source of spiritual insight, and an extended allegory.
https://en.wikipedia.org/wiki/Journey_to_the_West
| Tehachapi Mountains | |
|---|---|
 Tehachapi Mountains Crest peaks | |
| Highest point | |
| Peak | Double Mountain |
| Elevation | 7,981 ft (2,433 m) |
| Dimensions | |
| Length | 40 mi (64 km) |
| Geography | |
| Country | United States |
| State | California |
| Counties | Kern and Los Angeles |
| Range coordinates | 34°57′N 118°35′WCoordinates: 34°57′N 118°35′W |
| Parent range | Transverse Ranges |
| Borders on | Sierra Nevada, San Emigdio Mountains and Sierra Pelona Mountains |
The Tehachapi Mountains (/təˈhætʃəpi/; Kawaiisu: Tihachipia, meaning "hard climb")[1][2] are a mountain range in the Transverse Ranges system of California in the Western United States. The range extends for approximately 40 miles (64 km) in southern Kern County and northwestern Los Angeles County and form part of the boundary between the San Joaquin Valley and the Mojave Desert.
https://en.wikipedia.org/wiki/Tehachapi_Mountains
Mesquite Flat Sand Dunes
The Mesquite Flat Sand Dunes are at the northern end of the valley floor and are nearly surrounded by mountains on all sides. Due to their easy access from the road and the overall proximity of Death Valley to Hollywood, these dunes have been used to film sand dune scenes for several movies including films in the Star Wars series. The largest dune is called Star Dune and is relatively stable and stationary because it is at a point where the various winds that shape the dunes converge. The depth of the sand at its crest is 130–140 feet (40–43 metres) but this is small compared to other dunes in the area that have sand depths of up to 600–700 feet (180–210 metres) deep.
The primary source of the dune sands is probably the Cottonwood Mountains which lie to the north and northwest. The tiny grains of quartz and feldspar that form the sinuous sculptures that make up this dune field began as much larger pieces of solid rock.
In between many of the dunes are stands of creosote bush and some mesquite on the sand and on dried mud, which used to cover this part of the valley before the dunes intruded (mesquite was the dominant plant here before the sand dunes but creosote does much better in the sand dune conditions).
https://en.wikipedia.org/wiki/Places_of_interest_in_the_Death_Valley_area#Mesquite_Flat_Sand_Dunes
| Fletcher's frog | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Amphibia |
| Order: | Anura |
| Family: | Limnodynastidae |
| Genus: | Platyplectrum |
| Species: | P. fletcheri
|
| Binomial name | |
| Platyplectrum fletcheri (Boulenger, 1890)
| |

| |
| Range of the Fletcher's frog | |
| Synonyms | |
| |
Fletcher's frog (Platyplectrum fletcheri), commonly known as the sandpaper frog or black-soled frog, is a species of nocturnal, terrestrial frog native to eastern Australia.[2] It is primarily found in wet sclerophyll forests along mountain ranges and the coast.[3]
The Fletcher's frog's breeding behavior revolves around ephemeral water bodies created by rainfall. Male frogs compete with one another over territories that contain these pools, while female frogs choose desirable males to mate with. Female frogs produce foam and lay their eggs within the frothy mass. The nest's mucus has protective properties that enhance the survival odds of the progeny.[4]
Because ephemeral environments are resource-poor, sandpaper frog tadpoles rely primarily on the cannibalism of conspecific eggs to satisfy their nutritional needs. The Fletcher's frog's post-metamorphic life revolves around foraging for food in leaf litter and searching for mates.[5]
The Fletcher's frog is semelparous species, mating and laying an abundance of eggs after a courtship opportunity that might arise once in a lifetime.[6]
https://en.wikipedia.org/wiki/Fletcher%27s_frog
This is a list of major mountain peaks in the U.S. State of Colorado.
This article comprises three sortable tables of major mountain peaks[a] in Colorado.
The summit of a mountain or hill may be measured in three principal ways:
- The topographic elevation of a summit measures the height of the summit above a geodetic sea level.[b][c] The first table below ranks the 55 highest major summits of Colorado by elevation.
- The topographic prominence of a summit is a measure of how high the summit rises above its surroundings.[d][c] The second table below ranks the 50 most prominent summits of Colorado.
- The topographic isolation (or radius of dominance) of a summit measures how far the summit lies from its nearest point of equal elevation.[e] The third table below ranks the 50 most isolated major summits of Colorado.
https://en.wikipedia.org/wiki/List_of_mountain_peaks_of_Colorado
The Skeleton Coast is the northern part of the Atlantic coast of Namibia and south of Angola from the Kunene River south to the Swakop River, although the name is sometimes used to describe the entire Namib Desert coast. The indigenous San people (formerly known as Bushmen), of the Namibian interior called the region "The Land God Made in Anger", while Portuguese sailors once referred to it as "The Gates of Hell".
On the coast, the upwelling of the cold Benguela current gives rise to dense ocean fogs (called cassimbo by the Angolans) for much of the year. The winds blow from land to sea, rainfall rarely exceeds 10 millimetres (0.39 in) annually, and the climate is highly inhospitable. There is a constant, heavy surf on the beaches. In the days before engine-powered ships and boats, it was possible to get ashore through the surf but impossible to launch from the shore. The only way out was by going through a marsh hundreds of kilometres long and only accessible via a hot and arid desert.
The coast is largely soft sand occasionally interrupted by rocky outcrops. The southern section consists of gravel plains, while north of Terrace Bay the landscape is dominated by high sand dunes. Skeleton Bay is now known as a great location for surfing. The Saltyjackal, a surf camp located in Swakopmund, Namibia, is currently the only group that runs guided surf trips along the Skeleton Coast.[2]
https://en.wikipedia.org/wiki/Skeleton_Coast
List of mountain ranges of Oregon
There are at least 50 named mountain ranges in the U.S. state of Oregon. Many of these ranges extend into the neighboring states of California, Idaho, Nevada, and Washington. Elevations and coordinates are from the U.S. Geological Survey, Geographic Names Information System, unless otherwise indicated.
See also
- List of mountains of Oregon
- Lists of Oregon-related topics
- List of mountain ranges of California
- List of mountain ranges of Nevada
Notes
- "White Horse Mountains". Geographic Names Information System. United States Geological Survey.
https://en.wikipedia.org/wiki/List_of_mountain_ranges_of_Oregon
| Guadalupe Mountains National Park | |
|---|---|
 Guadalupe Peak, the highest point in Texas, as seen from Hunter Peak | |
| Location | Culberson County and Hudspeth County, Texas, United States |
| Nearest city | Dell City, Texas |
| Coordinates | 31°55′N 104°52′WCoordinates: 31°55′N 104°52′W |
| Area | 86,367 acres (349.51 km2)[1] |
| Established | September 30, 1972 |
| Visitors | 172,347 (in 2018)[2] |
| Governing body | National Park Service |
| Website | Guadalupe Mountains National Park |
Guadalupe Mountains National Park is an American national park in the Guadalupe Mountains, east of El Paso, Texas. The mountain range includes Guadalupe Peak, the highest point in Texas at 8,751 feet (2,667 m), and El Capitan used as a landmark by travelers on the route later followed by the Butterfield Overland Mail stagecoach line. The ruins of a stagecoach station stand near the Pine Springs visitor center. The restored Frijole Ranch contains a small museum of local history and is the trailhead for Smith Spring. The park covers 86,367 acres (134.9 sq mi; 349.5 km2)[1] in the same mountain range as Carlsbad Caverns National Park, about 25 miles (40 km) to the north in New Mexico. The Guadalupe Peak Trail winds through pinyon pine and Douglas-fir forests as it ascends over 3,000 feet (910 m) to the summit of Guadalupe Peak, with views of El Capitan and the Chihuahuan Desert.
The McKittrick Canyon trail leads to a stone cabin built in the early 1930s as the vacation home of Wallace Pratt, a petroleum geologist who donated the land. Dog Canyon, on the northern park boundary at the Texas-New Mexico State line, is accessed via Carlsbad, New Mexico or Dell City, Texas. Camping is available at the Pine Springs campground and at Dog Canyon. A public corral for livestock is available by reservation. The park observes Mountain Time.
The Gypsum sand dunes lie on the west side of the park near Dell City.[3] A rough four-wheel drive road leads to the Williams Ranch.[4]
https://en.wikipedia.org/wiki/Guadalupe_Mountains_National_Park
| Ural | |
|---|---|
 | |
 | |
| Location | |
| Countries | Kazakhstan, Russia |
| Cities | Magnitogorsk, Orsk, Novotroitsk, Orenburg, Oral, Atyrau |
| Physical characteristics | |
| Source | |
| • location | Ural Mountains |
| Mouth | Caspian Sea |
• coordinates | 46°53′N 51°37′ECoordinates: 46°53′N 51°37′E |
| Length | 2,428 km (1,509 mi) |
| Basin size | 231,000 km2 (89,000 sq mi) |
| Discharge | |
| • average | 400 m3/s (14,000 cu ft/s) |
| Official name | Ural River Delta and adjacent Caspian Sea coast |
| Designated | 10 March 2009 |
| Reference no. | 1856[1] |
The Ural (Russian: Урал, pronounced [ʊˈraɫ]), known before 1775 as Yaik (Russian: Яик, Bashkir: Яйыҡ, romanized: Yayıq, pronounced [jɑˈjɯq]; Kazakh: Жайық, romanized: Jaiyq, pronounced [ʑɑˈjəq]), is a river flowing through Russia and Kazakhstan in the continental border between Europe and Asia. It originates in the southern Ural Mountains and discharges into the Caspian Sea. At 2,428 kilometres (1,509 mi), it is the third-longest river in Europe after the Volga and the Danube, and the 18th-longest river in Asia. The Ural is conventionally considered part of the boundary between the continents of Europe and Asia.
The Ural rises near Mount Kruglaya in the Ural Mountains, flows south parallel and west of the north-flowing Tobol, through Magnitogorsk, and around the southern end of the Urals, through Orsk where it turns west for about 300 kilometres (190 mi), to Orenburg, where the river Sakmara joins. From Orenburg it continues west, passing into Kazakhstan, then turning south again at Oral, and meandering through a broad flat plain until it reaches the Caspian a few miles below Atyrau, where it forms a fine 'digitate' (tree-like) delta.[2]
https://en.wikipedia.org/wiki/Ural_(river)
| Mount Bromo | |
|---|---|
 View of Mts. Bromo, Semeru, Batok, and Widodaren, Tengger Caldera | |
| Highest point | |
| Elevation | 2,329 m (7,641 ft)[1] |
| Listing | Spesial Ribu |
| Coordinates | 7°56′30″S 112°57′00″ECoordinates: 7°56′30″S 112°57′00″E[1] |
| Geography | |
| Geology | |
| Mountain type | Somma volcano |
| Last eruption | 21 March 2019 (ongoing)[2] |
The Bromo (Javanese: ꦧꦿꦩ), or Mount Bromo (Javanese: ꦒꦸꦤꦸꦁꦧꦿꦩ Pegon: ڮنڠ برومو, romanized: Gunung Bromo) is an active somma volcano and part of the Tengger mountains, in East Java, Indonesia. At 2,329 meters (7,641 ft) it is not the highest peak of the massif, but the most famous. The area is one of the most visited tourist destinations in East Java, and the volcano is included in the Bromo Tengger Semeru National Park. The name Bromo comes from the Javanese pronunciation of Brahma, the Hindu god of creation. At the mouth of the crater, there is an idol of Ganesha, the Hindu god of wisdom which is being worshipped by the Javanese Hindus.[3] Mount Bromo is located in the middle of a plain called "Sea of Sand" (Javanese: Segara Wedi or Indonesian: Lautan Pasir), a nature reserve that has been protected since 1919.
A typical way to visit Mount Bromo is from the nearby mountain village of Cemoro Lawang. From there it is possible to walk to the volcano in about 45 minutes, but it is also possible to take an organized jeep tour, including stops at the viewpoint of Mount Penanjakan (2,770 m (9,090 ft)) (Indonesian: Gunung Penanjakan). The sights on Mount Penanjakan can also be reached on foot in about two hours. Depending on the level of volcanic activity, the Indonesian Center for Volcanology and Disaster Mitigation sometimes issues a warning not to visit Mount Bromo.
https://en.wikipedia.org/wiki/Mount_Bromo
| Horse Ranch Mountain | |
|---|---|
 Horse Ranch Mountain, as seen from the Taylor Creek area, August 2004 | |
| Highest point | |
| Elevation | 8,733 ft (2,662 m)[1] |
| Prominence | 966 feet (294 m) |
| Coordinates | 37°28′40″N 113°9′30″W[2] |
| Geography | |
| Location | Zion National Park in Washington County, Utah, United States |
| Parent range | Colorado Plateau |
| Topo map | USGS Kolob Arch |
Horse Ranch Mountain is an 8,733-foot (2,662 m) mountain in the Kolob Canyons section of Zion National Park in northeastern Washington County, Utah, United States,[1] that is the highest summit within the national park.[3] It rises above Camp Creek to the north and Taylor Creek to the south. Its neighbors include Tucupit Point, 1 mi (1.6 km) to the south, and Timber Top Mountain is situated 3.9 mi (6.3 km) to the south-southwest.
https://en.wikipedia.org/wiki/Horse_Ranch_Mountain
| Rock of Gibraltar | |
|---|---|
 Western face of the Rock of Gibraltar, in April 2006 | |
| Highest point | |
| Elevation | 426 m (1,398 ft) |
| Prominence | 423 m (1,388 ft)[1] |
| Coordinates | 36°07′28.1″N 05°20′35.2″WCoordinates: 36°07′28.1″N 05°20′35.2″W[2] |
| Geography | |
| Location | Gibraltar |
| Parent range | Betic Cordillera |
| Geology | |
| Age of rock | Jurassic |
| Climbing | |
| Easiest route | Gibraltar Cable Car, Road, Hike |
The Rock of Gibraltar (from the Arabic name Jabel-al-Tariq) is a monolithic limestone promontory located in the British territory of Gibraltar, near the southwestern tip of Europe on the Iberian Peninsula, and near the entrance to the Mediterranean.[3] It is 426 m (1,398 ft) high. Most of the Rock's upper area is covered by a nature reserve, which is home to around 300 Barbary macaques. These macaques, as well as a labyrinthine network of tunnels, attract many tourists each year.
The Rock of Gibraltar, one of the two traditional Pillars of Hercules, was known to the Romans as Mons Calpe, the other pillar being Mons Abila, either Monte Hacho or Jebel Musa on the African side of the Strait. According to ancient myths fostered by the Greeks and the Phoenicians,[3] and later perpetuated by the Romans,[4] the two points marked the limit to the known world, although the Phoenicians had actually sailed beyond this point into the Atlantic, both northward and southward.[4]
The Mediterranean Sea surrounds Gibraltar.
https://en.wikipedia.org/wiki/Rock_of_Gibraltar
| Goitered gazelle | |
|---|---|

| |
| Male of the Persian subspecies (G. s. subgutturosa) at Korkeasaari Zoo in Helsinki | |

| |
| Female goitered gazelle at the Shirvan National Park, Azerbaijan | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Artiodactyla |
| Family: | Bovidae |
| Subfamily: | Antilopinae |
| Tribe: | Antilopini |
| Genus: | Gazella |
| Species: | G. subgutturosa
|
| Binomial name | |
| Gazella subgutturosa (Güldenstädt, 1780)
| |
| Subspecies | |

| |
The goitered gazelle (Gazella subgutturosa) or black-tailed gazelle is a gazelle native to Turkey, Georgia, Azerbaijan, Iran, parts of Iraq and Pakistan, Afghanistan, Tajikistan, Kyrgyzstan, Uzbekistan, Turkmenistan, Kazakhstan and in northwestern China and Mongolia.[1] The specific name, meaning "full below the throat", refers to the male having an enlargement of the neck and throat during the mating season.
https://en.wikipedia.org/wiki/Goitered_gazelle
| Arabian Desert ٱلصَّحْرَاء ٱلْعَرَبِيَّة | |
|---|---|
 Desert near Sharjah, United Arab Emirates | |
 Map of the Arabian Desert ecoregion | |
| Ecology | |
| Realm | Palearctic |
| Biome | deserts and xeric shrublands |
| Borders | |
List | |
| Geography | |
|---|---|
| Area | 1,855,470[1] km2 (716,400 sq mi) |
| Countries | |
List | |
| Conservation | |
|---|---|
| Conservation status | critical/endangered[2] |
| Protected | 4.368%[1] |
The Arabian Desert (Arabic: ٱلصَّحْرَاء ٱلْعَرَبِيَّة) is a vast desert wilderness in Western Asia that occupies almost the entire Arabian Peninsula.[3] It stretches from Yemen to the Persian Gulf and Oman to Jordan and Iraq. It occupies most of the Arabian Peninsula, with an area of 2,330,000 square kilometers (900,000 sq mi). It is the fifth largest desert in the world, and the largest in Asia. At its center is Ar-Rub' al-Khali (The Empty Quarter), one of the largest continuous bodies of sand in the world. It is an extension of the Sahara Desert.[4]
https://en.wikipedia.org/wiki/Arabian_Desert
Kings Mountain, North Carolina | |
|---|---|
 The downtown of Kings Mountain along Battleground Avenue | |
| Motto: The Historical City | |
 Location of Kings Mountain, North Carolina | |
| Coordinates: 35°14′39″N 81°20′33″WCoordinates: 35°14′39″N 81°20′33″W | |
| Country | |
| State | |
| County | Cleveland, Gaston |
| Government | |
| • Type | Mayor-Council |
| • Mayor | Scott Neisler |
| • City Manager | Marilyn Sellers |
| Area | |
| • Total | 13.97 sq mi (36.19 km2) |
| • Land | 13.76 sq mi (35.65 km2) |
| • Water | 0.21 sq mi (0.54 km2) |
| Elevation | 1,007 ft (307 m) |
| Population (2020) | |
| • Total | 11,142 |
| • Density | 809.50/sq mi (312.55/km2) |
| Time zone | UTC−5 (Eastern (EST)) |
| • Summer (DST) | UTC−4 (EDT) |
| ZIP Code | 28086 |
| Area code | 704 |
| FIPS code | 37-35880[2] |
| GNIS feature ID | 0988003[3] |
| Website | www |
Kings Mountain is a small suburban city within the Charlotte metropolitan area in Cleveland and Gaston counties, North Carolina, United States. Most of the city is in Cleveland County, with a small eastern portion in Gaston County. The population was 10,296 at the 2010 census.[4]
https://en.wikipedia.org/wiki/Kings_Mountain,_North_Carolina
 | |
| Location | Aswan Governorate, Egypt |
|---|---|
| Region | Nubia |
| Coordinates | 22°20′13″N 31°37′32″ECoordinates: 22°20′13″N 31°37′32″E |
| Type | Temple |
| History | |
| Builder | Ramesses II |
| Founded | Approximately 1264 BC |
| Periods | New Kingdom of Egypt |
| Official name | Nubian Monuments from Abu Simbel to Philae |
| Type | Cultural |
| Criteria | i, iii, vi |
| Designated | 1979 (3rd session) |
| Reference no. | 88 |
| Region | Arab States |
Abu Simbel is a historic site comprising two massive rock-cut temples in the village of Abu Simbel (Arabic: أبو سمبل), Aswan Governorate, Upper Egypt, near the border with Sudan. It is situated on the western bank of Lake Nasser, about 230 km (140 mi) southwest of Aswan (about 300 km (190 mi) by road). The twin temples were originally carved out of the mountainside in the 13th century BC, during the 19th Dynasty reign of the Pharaoh Ramesses II. Their huge external rock relief figures of Ramesses II have become iconic. His wife, Nefertari, and children can be seen in smaller figures by his feet. Sculptures inside the Great Temple commemorate Ramesses II's heroic leadership at the Battle of Kadesh.
The complex was relocated in its entirety in 1968 to higher ground to avoid it being submerged by Lake Nasser, the Aswan Dam reservoir. As part of International Campaign to Save the Monuments of Nubia, an artificial hill was made from a domed structure to house the Abu Simbel Temples, under the supervision of a Polish archaeologist, Kazimierz Michałowski, from the Polish Centre of Mediterranean Archaeology University of Warsaw.[1][2]
The Abu Simbel complex, and other relocated temples from Nubian sites such as Philae, Amada, Wadi es-Sebua, are part of the UNESCO World Heritage Site known as the "Nubian Monuments".[2]
https://en.wikipedia.org/wiki/Abu_Simbel
The Red Desert is a high-altitude desert and sagebrush steppe located in the south-central portion of the U.S. state of Wyoming, comprising approximately 9,320 square miles (24,100 square kilometers). Among the natural features in the Red Desert region are the Great Divide Basin, a unique endorheic drainage basin formed by a division in the Continental Divide, and the Killpecker Sand Dunes, the largest living dune system in the United States. In the 19th century, the Oregon, California, and Mormon Trails crossed the Continental Divide at South Pass, just north of the Red Desert. Today, busy Interstate 80 bisects the desert's southern region while gas field roads cross the desert.
The majority of the Red Desert is public land managed by the Rock Springs and Rawlins field offices of the U.S. Bureau of Land Management (BLM). The region is rich in oil, natural gas, uranium, and coal. An estimated 84% of the Red Desert has been "industrialized" by oil and gas drilling or by mining operations and associated roads.[1]
The Red Desert supports an abundance of wildlife, despite its scarcity of water and vegetation. The largest migratory herd of pronghorn in the lower 48 states and a rare desert elk herd, said to be the world's largest, live in the desert.[2] Ponds fed by summer snowmelt attract a wide range of migratory birds such as ducks, trumpeter swans,[citation needed] and white pelicans.[citation needed] Herds of wild, free-roaming horses protected under the Wild Free-Roaming Horses and Burros Act of 1971 roam the area, despite roundups and population control efforts by the BLM. Bison were once common as well and their skulls and horns can occasionally be found there.
https://en.wikipedia.org/wiki/Red_Desert_(Wyoming)
The Black Stallion, known as the Black or Shêtân, is the title character from author Walter Farley's bestselling series about the Arab stallion and his young owner, Alec Ramsay. The series chronicles the story of a Sheikh's prized stallion after he comes into Alec's possession through a ship journey gone awry. Later books in the series furnish the Black's backstory. Shaytan (under various transliterations) is the Arabic word for "devil".
The first book in the series, published in 1941, is titled The Black Stallion. The subsequent novels are about the Black himself and the stallion's three main offspring: his firstborn colt, Satan; his second colt, Bonfire; and his firstborn filly, Black Minx. Along with the Black, the series introduces a second stallion that is considered the Black's only equal - The Island Stallion, Flame. This is a separate storyline until Flame and the Black meet in two books - The Black Stallion and Flame, and The Black Stallion Challenged. However, news of Flame's win in an international race in Cuba, and his mysterious disappearance, are mentioned at the end of The Black Stallion Mystery, which serves as the first introduction of this rival for the later books in which they meet.
The first two books, as well as the final book of the series, were adapted for the films The Black Stallion (1979), The Black Stallion Returns (1983), and The Young Black Stallion (2003).
The Black Stallion was described as "the most famous fictional horse of the century" by The New York Times.[1]
https://en.wikipedia.org/wiki/The_Black_Stallion
The Dust Bowl was a period of severe dust storms that greatly damaged the ecology and agriculture of the American and Canadian prairies during the 1930s. The phenomenon was caused by a combination of both natural factors (severe drought) and manmade factors (a failure to apply dryland farming methods to prevent wind erosion, most notably the destruction of the natural topsoil by settlers in the region).[1][2] The drought came in three waves: 1934, 1936, and 1939–1940, but some regions of the High Plains experienced drought conditions for as many as eight years.[3]
The Dust Bowl has been the subject of many cultural works, notably the novel The Grapes of Wrath (1939) by John Steinbeck, the folk music of Woody Guthrie, and photographs depicting the conditions of migrants by Dorothea Lange, particularly the Migrant Mother, taken in 1936.
https://en.wikipedia.org/wiki/Dust_Bowl
| Florida black bear | |
|---|---|

| |
| A Florida black bear in the Ocala National Forest | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Carnivora |
| Family: | Ursidae |
| Genus: | Ursus |
| Species: | |
| Subspecies: | U. a. floridanus
|
| Trinomial name | |
| Ursus americanus floridanus Merriam, 1896
| |
| |
| Florida black bear range[2] | |
The Florida black bear (Ursus americanus floridanus) is a subspecies of the American black bear that has historically ranged throughout most of Florida and the southern portions of Georgia, Alabama, and Mississippi. The large black-furred bears live mainly in forested areas and have seen recent habitat reduction throughout the state due to increased human development, as well as habitat modifications within bear habitat.
https://en.wikipedia.org/wiki/Florida_black_bear
The sandhill crane (Antigone canadensis) is a species of large crane of North America and extreme northeastern Siberia. The common name of this bird refers to habitat like that at the Platte River, on the edge of Nebraska's Sandhills on the American Great Plains. Sandhill Cranes are known to hangout at the edges of bodies of water especially in the Central Florida region. The central Platte River valley in Nebraska is the most important stopover area for the nominotypical subspecies, the lesser sandhill crane (A. c. canadensis), with up to 450,000 of these birds migrating through annually.[3][4]
https://en.wikipedia.org/wiki/Sandhill_crane
| Barn swallow | |
|---|---|

| |
| H. r. rustica at Bygholm Vejle, Denmark | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Aves |
| Order: | Passeriformes |
| Family: | Hirundinidae |
| Genus: | Hirundo |
| Species: | H. rustica
|
| Binomial name | |
| Hirundo rustica | |
| Subspecies | |
|
6, see text | |

| |
| Range of H. rustica Breeding Resident Passage Non-breeding
| |
| Synonyms | |
| |
The barn swallow (Hirundo rustica) is the most widespread species of swallow in the world. It appears to have the largest natural distribution of any of the world's passerines, ranging over 251 million square kilometres globally. It is a distinctive passerine bird with blue upperparts and a long, deeply forked tail. It is found in Europe, Asia, Africa and the Americas. In Anglophone Europe it is just called the swallow; in northern Europe it is the only common species called a "swallow" rather than a "martin".
There are six subspecies of barn swallow, which breed across the Northern Hemisphere. Four are strongly migratory, and their wintering grounds cover much of the Southern Hemisphere as far south as central Argentina, the Cape Province of South Africa, and northern Australia. Its huge range means that the barn swallow is not endangered, although there may be local population declines due to specific threats.
The barn swallow is a bird of open country that normally uses man-made structures to breed and consequently has spread with human expansion. It builds a cup nest from mud pellets in barns or similar structures and feeds on insects caught in flight. This species lives in close association with humans, and its insect-eating habits mean that it is tolerated by humans; this acceptance was reinforced in the past by superstitions regarding the bird and its nest. There are frequent cultural references to the barn swallow in literary and religious works due to both its living in close proximity to humans and its annual migration. The barn swallow is the national bird of Austria and Estonia.
https://en.wikipedia.org/wiki/Barn_swallow
Gary | |
|---|---|
From top, left to right: City Hall (left) and Superior Courthouse (right), City Methodist Church, Knights of Columbus Building, Genesis Convention Center, 2300 Jackson Street in Midtown, and Gary Public Library & Cultural Center | |
| Nicknames: "City in Motion", "City of the Century", "Magic City", "Steel City", "City on the Move" | |
| Motto: We Are Doing Great Things | |
 Location of Gary in Lake County, Indiana | |
| Coordinates: 41°35′44″N 87°20′43″WCoordinates: 41°35′44″N 87°20′43″W[1] | |
| Country | United States |
| State | Indiana |
| County | Lake |
| Townships | Calumet, Hobart |
| Incorporated | July 14, 1906 |
| Named for | Elbert Henry Gary |
| Government | |
| • Type | Strong mayor–council |
| • Body | City council |
| • Mayor | Jerome A. Prince (D) |
| • City Clerk | Suzette Raggs (D) |
| • City Judge | Deidre L. Monroe (D) |
| Area | |
| • Total | 50.60 sq mi (131.05 km2) |
| • Land | 49.87 sq mi (129.15 km2) |
| • Water | 0.73 sq mi (1.89 km2) |
| Elevation | 607 ft (185 m) |
| Population (2020) | |
| • Total | 69,093 |
| • Density | 1,385.55/sq mi (534.97/km2) |
| Time zone | UTC−6 (Central) |
| • Summer (DST) | UTC−5 (Central) |
| ZIP Codes | 46401–46411 |
| Area code | 219 |
| FIPS code | 18-27000 |
| GNIS feature ID | 2394863[1] |
| Website | Official website |
Gary is a city in Lake County, Indiana, United States. The city has been historically dominated by major industrial activity and is home to U.S. Steel's Gary Works, the largest steel mill complex in North America. Gary is located along the southern shore of Lake Michigan about 25 miles (40 km) east of downtown Chicago, Illinois. The city is adjacent to the Indiana Dunes National Park, and is within the Chicago metropolitan area.[4][5]
https://en.wikipedia.org/wiki/Gary,_Indiana
| Boyne Mountain Resort | |
|---|---|
 | |
| Location | Boyne Valley Township, Charlevoix County |
| Nearest major city | Boyne Falls, Michigan |
| Coordinates | 45°09′50″N 84°55′58″WCoordinates: 45°09′50″N 84°55′58″W |
| Status | Operating |
| Owner | Boyne Resorts |
| Vertical | 500 ft (150 m) |
| Top elevation | 1,120 ft (340 m) |
| Base elevation | 620 ft (190 m) |
| Skiable area | 450 acres (1.8 km2) |
| Runs | 60 |
| Lift capacity | 22,650 |
| Terrain parks | Yes, 4 |
| Snowmaking | Yes, 90% |
| Night skiing | Yes |
| Website | Boyne.com |
Boyne Mountain Resort is a ski resort with a collection of accommodations in Northern Michigan located near Boyne City operated by Boyne Resorts. The center piece is an upscale resort called The Mountain Grand Lodge and Spa.[1]
Boyne Mountain has continued use of the first chairlift built, constructed by the Union Pacific Railroad in 1936 for use at its new resort in Sun Valley, Idaho. It is also the location of Avalanche Bay, the largest indoor water park in Michigan.[2] Boyne Mountain is the sister resort of Boyne Highlands.[3]
https://en.wikipedia.org/wiki/Boyne_Mountain_Resort
The Schwarzwaldhochstraße or Black Forest High Road is the oldest and one of the best known themed drives in Germany. It is a part of the B 500 federal highway and leads over 60 km from Baden-Baden to Freudenstadt.[1]
https://en.wikipedia.org/wiki/Schwarzwaldhochstra%C3%9Fe
| Mount Rushmore National Memorial | |
|---|---|
 Mount Rushmore with Gutzon Borglum's sculpted heads of George Washington, Thomas Jefferson, Theodore Roosevelt and Abraham Lincoln (left to right) | |
| Location | Pennington County, South Dakota |
| Nearest city | Keystone, South Dakota |
| Coordinates | 43°52′44″N 103°27′35″WCoordinates: 43°52′44″N 103°27′35″W |
| Area | 1,278 acres (5.17 km2) |
| Authorized | March 3, 1925 |
| Visitors | 2,074,986 (in 2020)[1] |
| Governing body | National Park Service |
| Website | www |
Mount Rushmore National Memorial | |
| Built | 1927–1941 |
| Architect | Gutzon and Lincoln Borglum |
| NRHP reference No. | 66000718 |
| Added to NRHP | October 15, 1966 |
The Mount Rushmore National Memorial is a national memorial centered on a colossal sculpture carved into the granite face of Mount Rushmore (Lakota: Tȟuŋkášila Šákpe, or Six Grandfathers) in the Black Hills near Keystone, South Dakota, United States. Sculptor Gutzon Borglum designed the sculpture, called Shrine of Democracy,[2] and oversaw the project's execution from 1927 to 1941 with the help of his son, Lincoln Borglum.[3][4] The sculpture features the 60-foot-tall (18 m) heads of four United States presidents: George Washington (1732–1799), Thomas Jefferson (1743–1826), Theodore Roosevelt (1858–1919) and Abraham Lincoln (1809–1865).[5] Mount Rushmore attracts more than two million visitors annually.[1] The four presidents were chosen to represent the nation's birth, growth, development and preservation, respectively.[6] The memorial park covers 1,278 acres (2.00 sq mi; 5.17 km2)[7] and the mountain's elevation is 5,725 feet (1,745 m) above sea level.[8]
https://en.wikipedia.org/wiki/Mount_Rushmore
| Brecon Beacons | |
|---|---|
| Welsh: Bannau Brycheiniog | |
IUCN category V (protected landscape/seascape) | |
| Location | Powys, Wales (Brecon Beacons National Park) |
The Brecon Beacons (Welsh: Bannau Brycheiniog, [ˈbanai̯ brəˈχəi̯njɔɡ] (![]() listen)) is a mountain range in South Wales.[1] In a narrow sense, the name refers to the range of Old Red Sandstone peaks which lie to the south of Brecon. Sometimes referred to as 'the central Beacons' they include South Wales' highest mountain, Pen y Fan.[2] The range forms the central section of the Brecon Beacons National Park (Parc Cenedlaethol Bannau Brycheiniog),
a designation which also encompasses ranges both to the east and the
west of 'the central Beacons'. This much wider area is also commonly
referred to as 'the Brecon Beacons', and it includes the Black Mountains to the east as well as the similarly named but quite distinct Black Mountain to the west. The highest peaks include Fan Brycheiniog to the west and Pen y Fan
in the central part. They share the same basic geology as the central
range, and so exhibit many similar features, such as the north-facing
escarpment and glacial features such as lakes and cwms (cirques).
listen)) is a mountain range in South Wales.[1] In a narrow sense, the name refers to the range of Old Red Sandstone peaks which lie to the south of Brecon. Sometimes referred to as 'the central Beacons' they include South Wales' highest mountain, Pen y Fan.[2] The range forms the central section of the Brecon Beacons National Park (Parc Cenedlaethol Bannau Brycheiniog),
a designation which also encompasses ranges both to the east and the
west of 'the central Beacons'. This much wider area is also commonly
referred to as 'the Brecon Beacons', and it includes the Black Mountains to the east as well as the similarly named but quite distinct Black Mountain to the west. The highest peaks include Fan Brycheiniog to the west and Pen y Fan
in the central part. They share the same basic geology as the central
range, and so exhibit many similar features, such as the north-facing
escarpment and glacial features such as lakes and cwms (cirques).
https://en.wikipedia.org/wiki/Brecon_Beacons
A cirque (French: [siʁk]; from the Latin word circus) is an amphitheatre-like valley formed by glacial erosion. Alternative names for this landform are corrie (from Scottish Gaelic: coire, meaning a pot or cauldron)[1] and cwm (Welsh for 'valley'; pronounced [kʊm]). A cirque may also be a similarly shaped landform arising from fluvial erosion.
The concave shape of a glacial cirque is open on the downhill side, while the cupped section is generally steep. Cliff-like slopes, down which ice and glaciated debris combine and converge, form the three or more higher sides. The floor of the cirque ends up bowl-shaped, as it is the complex convergence zone of combining ice flows from multiple directions and their accompanying rock burdens. Hence, it experiences somewhat greater erosion forces and is most often overdeepened below the level of the cirque's low-side outlet (stage) and its down-slope (backstage) valley. If the cirque is subject to seasonal melting, the floor of the cirque most often forms a tarn (small lake) behind a dam, which marks the downstream limit of the glacial overdeepening. The dam itself can be composed of moraine, glacial till, or a lip of the underlying bedrock.[2]
The fluvial cirque or makhtesh, found in karst landscapes, is formed by intermittent river flow cutting through layers of limestone and chalk leaving sheer cliffs. A common feature for all fluvial-erosion cirques is a terrain which includes erosion resistant upper structures overlying materials which are more easily eroded.
https://en.wikipedia.org/wiki/Cirque
| Mount Bromo | |
|---|---|
 View of Mts. Bromo, Semeru, Batok, and Widodaren, Tengger Caldera | |
| Highest point | |
| Elevation | 2,329 m (7,641 ft)[1] |
| Listing | Spesial Ribu |
| Coordinates | 7°56′30″S 112°57′00″ECoordinates: 7°56′30″S 112°57′00″E[1] |
| Geography | |
| Geology | |
| Mountain type | Somma volcano |
| Last eruption | 21 March 2019 (ongoing)[2] |
The Bromo (Javanese: ꦧꦿꦩ), or Mount Bromo (Javanese: ꦒꦸꦤꦸꦁꦧꦿꦩ Pegon: ڮنڠ برومو, romanized: Gunung Bromo) is an active somma volcano and part of the Tengger mountains, in East Java, Indonesia. At 2,329 meters (7,641 ft) it is not the highest peak of the massif, but the most famous. The area is one of the most visited tourist destinations in East Java, and the volcano is included in the Bromo Tengger Semeru National Park. The name Bromo comes from the Javanese pronunciation of Brahma, the Hindu god of creation. At the mouth of the crater, there is an idol of Ganesha, the Hindu god of wisdom which is being worshipped by the Javanese Hindus.[3] Mount Bromo is located in the middle of a plain called "Sea of Sand" (Javanese: Segara Wedi or Indonesian: Lautan Pasir), a nature reserve that has been protected since 1919.
A typical way to visit Mount Bromo is from the nearby mountain village of Cemoro Lawang. From there it is possible to walk to the volcano in about 45 minutes, but it is also possible to take an organized jeep tour, including stops at the viewpoint of Mount Penanjakan (2,770 m (9,090 ft)) (Indonesian: Gunung Penanjakan). The sights on Mount Penanjakan can also be reached on foot in about two hours. Depending on the level of volcanic activity, the Indonesian Center for Volcanology and Disaster Mitigation sometimes issues a warning not to visit Mount Bromo.
https://en.wikipedia.org/wiki/Mount_Bromo
| Andean mountain cat | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Carnivora |
| Suborder: | Feliformia |
| Family: | Felidae |
| Subfamily: | Felinae |
| Genus: | Leopardus |
| Species: | L. jacobita[1]
|
| Binomial name | |
| Leopardus jacobita[1] (Cornalia, 1865)
| |

| |
| Distribution of the Andean cat, 2016[2] | |
| Synonyms | |
|
Oreailurus jacobita | |
The Andean mountain cat (Leopardus jacobita) is a small wild cat native to the high Andes that has been listed as Endangered on the IUCN Red List because fewer than 1,500 individuals are thought to exist in the wild.[2] It is traditionally considered a sacred animal by indigenous Aymara and Quechua people.[3]
The Andean mountain cat was first described by Emilio Cornalia who named it in honor of Jacobita Mantegazza. It is a monotypic species.[4]
https://en.wikipedia.org/wiki/Andean_mountain_cat
Saguache County | |
|---|---|
 Entering Saguache County from the north on U.S. 285 | |
 Location within the U.S. state of Colorado | |
 Colorado's location within the U.S. | |
| Coordinates: 38°05′N 106°18′W | |
| Country | |
| State | |
| Founded | December 29, 1866 |
| Seat | Saguache |
| Largest town | Center |
| Area | |
| • Total | 3,170 sq mi (8,200 km2) |
| • Land | 3,169 sq mi (8,210 km2) |
| • Water | 1.7 sq mi (4 km2) 0.05%% |
| Population | |
| • Estimate (2020) | 6,368 |
| • Density | 2.0/sq mi (0.8/km2) |
| Time zone | UTC−7 (Mountain) |
| • Summer (DST) | UTC−6 (MDT) |
| Congressional district | 3rd |
| Website | saguachecounty |
Saguache County (suh-WATCH /səˈwɑːtʃ/ (![]() listen)) is a county located in the U.S. state of Colorado.[3] As of the 2020 census, the population was 6,368.[4] The county seat is Saguache.[5]
listen)) is a county located in the U.S. state of Colorado.[3] As of the 2020 census, the population was 6,368.[4] The county seat is Saguache.[5]
https://en.wikipedia.org/wiki/Saguache_County,_Colorado
Willard Bay is a man-made fresh water reservoir in the Great Salt Lake, in northern Utah. The bay was separated from the Great Salt Lake in 1964, and has since served as a source of irrigation water and recreation for the northern Wasatch Front metro area.
https://en.wikipedia.org/wiki/Willard_Bay
| Ore Mountains | |
|---|---|
| Erz Mountains Krušné Mountains | |
 Reservoir near Myslivny | |
| Highest point | |
| Peak | Klínovec |
| Elevation | 1,244 m (4,081 ft) |
| Coordinates | 50°23′46″N 12°58′04″E |
| Naming | |
| Native name |
|
| Geography | |
| Countries | Czech Republic and Germany |
| Regions/States | Karlovy Vary, Ústí nad Labem and Saxony |
| Range coordinates | 50°35′N 13°00′ECoordinates: 50°35′N 13°00′E |
| Geology | |
| Orogeny | Variscan |
| Age of rock | Paleozoic |
| Type of rock | sedimentary, metamorphic and igneous rocks |
| Official name | Erzgebirge/Krušnohoří Mining Region |
| Type | Cultural |
| Criteria | (ii), (iii), (iv) |
| Designated | 2019 |
| Reference no. | 1478 |
| Region | Western Europe/Eastern Europe |
The Ore Mountains lie along the Czech–German border, separating the historical regions of Bohemia in the Czech Republic and Saxony in Germany. The highest peaks are the Klínovec in the Czech Republic (German: Keilberg), which rises to 1,244 metres (4,081 ft) above sea level and the Fichtelberg in Germany (1,215 metres (3,986 ft)).
The nature of the Ore Mountains has been intensively shaped by human intervention and has created a diverse cultural landscape. In particular, mining with its tips, dams, ditches and sinkholes directly shaped the landscape and the habitats of plants and animals in many places. The region was also the setting of the earliest stages of the early modern transformation of mining and metallurgy from a craft to a large-scale industry, a process that preceded and enabled the later Industrial Revolution.
The higher altitudes from around 500 m above sea level on the German side belong to the Ore Mountains/Vogtland Nature Park – the largest of its kind in Germany with a length of 120 km. The eastern Ore Mountains are protected landscape. Other smaller areas on the German and Czech side are protected as nature reserves and natural monuments. On the ridges there are also several larger raised bogs that are only fed by rainwater. The mountains are popular for hiking and there are winter sports areas at higher elevations. In 2019, the region became a UNESCO World Heritage Site.[1]
https://en.wikipedia.org/wiki/Ore_Mountains
| Brown-throated martin | |
|---|---|

| |
| In Natal, South Africa | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Aves |
| Order: | Passeriformes |
| Family: | Hirundinidae |
| Genus: | Riparia |
| Species: | R. paludicola
|
| Binomial name | |
| Riparia paludicola (Vieillot, 1817)
| |
The brown-throated martin or brown-throated sand martin (Riparia paludicola) is a small passerine bird in the swallow family. It was first formally described as Hirundo paludicola by French ornithologist Louis Vieillot in 1817 in his Nouveau Dictionnaire d'Histoire Naturelle.[2] It was formerly regarded as conspecific with the grey-throated martin (R. chinensis) under the name "plain martin".
https://en.wikipedia.org/wiki/Brown-throated_martin
| Purple martin | |
|---|---|

| |
| Adult male | |

| |
| Adult female | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Aves |
| Order: | Passeriformes |
| Family: | Hirundinidae |
| Genus: | Progne |
| Species: | P. subis
|
| Binomial name | |
| Progne subis | |

| |
| Orange: breeding; yellow: migration; blue: nonbreeding | |
| Synonyms | |
| |
The purple martin (Progne subis) is a passerine bird in the swallow family Hirundinidae. It is the largest swallow in North America. Despite its name, the purple martin is not truly purple. The dark blackish-blue feathers have an iridescent sheen caused by the refraction of incident light[2] giving them a bright blue to navy blue or deep purple appearance. In some light they may even appear green in color.
Being migratory, their breeding range extends from central Alberta down through the eastern United States. Subspecies breed in Baja California, Arizona, and New Mexico. Most make a brief stopover in the Yucatán Peninsula or Cuba during pre-breeding migration to North America and during post-breeding migration before reaching their overwintering site in South America.[3]
They are known for their speed, agility, and their characteristic mix of rapid flapping and gliding flight pattern. When approaching their nesting site, they will dive from the sky at great speeds with their wings tucked, just like the peregrine falcon does when hunting smaller birds.
https://en.wikipedia.org/wiki/Purple_martin
Astolfo (also Astolpho, Estous, and Estouls) is a fictional character in the Matter of France where he is one of Charlemagne's paladins. He is the son of Otto, the King of England (possibly referring to Charles' contemporary Offa of Mercia), and is a cousin to Orlando and Rinaldo, and a descendant of Charles Martel. While Astolfo's name appeared in the Old French chanson de geste The Four Sons of Aymon, his first major appearance was in the anonymous early fourteenth-century Franco-Venetian epic poem La Prise de Pampelune.[1] He was subsequently a major character (typically humorous) in Italian Renaissance romance epics, such as Morgante by Luigi Pulci, Orlando Innamorato by Matteo Maria Boiardo, and Orlando Furioso by Ludovico Ariosto.
https://en.wikipedia.org/wiki/Astolfo
List of pines by region
This is a list of pine species by geographical distribution. For a taxonomy of the genus, see Pinus classification.
Old World
Mature Pinus pinea (stone pine); note umbrella-shaped canopy |
Pollen cones of Pinus pinea (stone pine) |
A red pine (Pinus resinosa) with exposed roots |
Young spring growth ("candles") on a loblolly pine |
Monterey pine bark |
Monterey pine cone on forest floor |
Whitebark pine in the Sierra Nevada |
Hartweg's pine forest in Mexico |
The bark of a pine in Tecpan, Guatemala |
A pine, probably P. pseudostrobus, in Guatemala |
Europe, Mediterranean, West Asia
- Pinus brutia - Turkish pine
- Pinus canariensis - Canary Island pine
- Pinus cembra - Swiss pine
- Pinus halepensis - Aleppo pine
- Pinus heldreichii - Bosnian pine
- Pinus mugo - Mountain pine
- Pinus nigra - European black pine, Austrian pine
- Pinus peuce - Macedonian pine
- Pinus pinaster - Maritime pine
- Pinus pinea - Stone pine
- Pinus sylvestris - Scots pine
East Asia, Southeast Asia
- Pinus amamiana - Yakushima white pine
- Pinus armandii - Chinese white pine
- Pinus bhutanica - Bhutan white pine
- Pinus bungeana - Lacebark pine
- Pinus dalatensis - Vietnamese white pine
- Pinus densata - Sikang pine
- Pinus densiflora - Korean red pine
- Pinus fenzeliana - Hainan white pine
- Pinus hwangshanensis - Huangshan pine
- Pinus kesiya - Khasi pine
- Pinus koraiensis - Korean pine
- Pinus krempfii - Krempf's pine
- Pinus latteri - Tenasserim pine
- Pinus luchuensis - Luchu pine
- Pinus massoniana - Masson's pine
- Pinus merkusii - Sumatran pine
- Pinus morrisonicola - Taiwan white pine
- Pinus parviflora - Japanese white pine
- Pinus pumila - Siberian dwarf pine
- Pinus roxburghii - Chir pine
- Pinus sibirica - Siberian pine
- Pinus squamata - Qiaojia pine
- Pinus tabuliformis - Chinese red pine
- Pinus taiwanensis - Taiwan red pine
- Pinus thunbergii - Japanese black pine
- Pinus wallichiana - Blue pine or Bhutan pine
- Pinus wangii (syn. P. kwangtungensis) - Guangdong white pine
- Pinus yunnanensis - Yunnan pine
New World
Eastern Canada, Eastern United States
- Pinus banksiana - Jack pine
- Pinus clausa - Sand pine
- Pinus echinata - Shortleaf pine
- Pinus elliottii - Slash pine
- Pinus glabra - Spruce pine
- Pinus palustris - Longleaf pine
- Pinus pungens - Table Mountain pine
- Pinus resinosa - Red pine
- Pinus rigida - Pitch pine
- Pinus serotina - Pond pine
- Pinus strobus - Eastern white pine
- Pinus taeda - Loblolly pine
- Pinus virginiana - Virginia pine
Western Canada, Western United States, Northern Mexico
- Pinus albicaulis - Whitebark pine
- Pinus aristata - Rocky Mountains bristlecone pine
- Pinus attenuata - Knobcone pine
- Pinus balfouriana - Foxtail pine
- Pinus contorta - Lodgepole pine
- Pinus coulteri - Coulter pine
- Pinus edulis - Colorado pinyon
- Pinus flexilis - Limber pine
- Pinus jeffreyi - Jeffrey pine
- Pinus lambertiana - Sugar pine
- Pinus longaeva - Great Basin bristlecone pine
- Pinus monophylla - Single-leaf pinyon
- Pinus monticola - Western white pine
- Pinus muricata - Bishop pine
- Pinus ponderosa (syn. P. washoensis) - Ponderosa pine
- Pinus radiata - Monterey pine, radiata pine
- Pinus remota - Texas pinyon, papershell pinyon
- Pinus sabineana - Gray pine, foothill pine, digger pine
- Pinus strobiformis - Southwestern white pine
- Pinus torreyana - Torrey pine
Southwestern United States, Mexico, Central America, Caribbean
- Pinus arizonica - Arizona pine
- Pinus ayacahuite - Mexican white pine
- Pinus caribaea - Caribbean pine
- Pinus cembroides - Mexican pinyon
- Pinus chiapensis - Chiapas white pine
- Pinus cooperi - Cooper's pine
- Pinus cubensis - Cuban pine
- Pinus culminicola - Potosi pinyon
- Pinus devoniana (syn. P. michoacana) - Michoacan pine
- Pinus durangensis - Durango pine
- Pinus engelmannii - Apache pine
- Pinus douglasiana - Douglas pine
- Pinus greggii - Gregg's pine
- Pinus hartwegii - Hartweg's pine
- Pinus herrerae - Herrera's pine
- Pinus jaliscana - Jalisco pine
- Pinus johannis - Johann's pinyon
- Pinus lawsonii - Lawson's pine
- Pinus leiophylla - Chihuahua pine
- Pinus lumholtzii - Lumholtz's pine
- Pinus luzmariae
- Pinus maximartinezii - Big-cone pinyon
- Pinus maximinoi (syn. P. tenuifolia) - Thinleaf pine
- Pinus montezumae - Montezuma pine
- Pinus nelsonii - Nelson's pinyon
- Pinus occidentalis - Hispaniolan pine
- Pinus oocarpa - Egg-cone pine
- Pinus patula - Patula pine
- Pinus orizabensis - Orizaba pinyon
- Pinus pinceana - Weeping pinyon
- Pinus praetermissa - McVaugh's pine
- Pinus pringlei - Pringle's pine
- Pinus pseudostrobus - Smooth-bark Mexican pine
- Pinus quadrifolia - Parry pinyon
- Pinus rzedowskii - Rzedowski's pine
- Pinus strobiformis - Chihuahua white pine
- Pinus tecunumanii - Tecun Uman pine
- Pinus teocote - Teocote pine
- Pinus tropicalis - Tropical pine
External links
Pinus by country.
https://en.wikipedia.org/wiki/List_of_pines_by_region
The Table Mountain Sandstone (TMS) is a group of rock formations within the Cape Supergroup sequence of rocks. Although the term "Table Mountain Sandstone" is still widely used in common parlance, the term TMS is no longer formally recognized; the correct name is the "Peninsula Formation Sandstone", which is part of the Table Mountain Group. The designation "Table Mountain Sandstone" will, however, in deference to the title, continue to be used in the rest of this article. The name is derived from the famous landmark in Cape Town, Table Mountain.
Table Mountain Sandstone is made up predominantly of quartzitic sandstone laid down between 510 (Cambrian Period) and 400 (Silurian Period) million years ago. It is the hardest, and most erosion resistant layer of the Cape Supergroup. It therefore forms most of the highest and most conspicuous peaks in the Western Cape, as well as the steepest cliffs of the Cape Fold Mountains, despite being the oldest, and, therefore, lowermost of the Cape Supergroup sequence.[2] The folding of the sequence into the parallel mountain ranges of the Western Cape started about 330 million years ago, affecting the Cape Supergroup from about Clanwilliam (approximately 200 km north of Cape Town), to about Port Elizabeth (approximately 650 km east of Cape Town). The Cape Supergroup sediments beyond these points are not folded into mountain ranges, but do, in places, form steep cliffs or gorges, where the surrounding sediments have been eroded away (see, for instance, Oribi Gorge in KwaZulu-Natal).[4][5]
https://en.wikipedia.org/wiki/Table_Mountain_Sandstone
| Midges | |
|---|---|

| |
| A biting midge feeding on blood through an artificial membrane for insect rearing | |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Suborder: | |
A midge is any small fly, including species in several families of non-mosquito Nematoceran Diptera. Midges are found (seasonally or otherwise) on practically every land area outside permanently arid deserts and the frigid zones. Some midges, such as many Phlebotominae (sand fly) and Simuliidae (black fly), are vectors of various diseases. Many others play useful roles as prey for insectivores, such as various frogs and swallows. Others are important as detritivores, and form part of various nutrient cycles. The habits of midges vary greatly from species to species, though within any particular family, midges commonly have similar ecological roles.
Examples of families that include species of midges include:[1]
- Blephariceridae, net-winged midges
- Cecidomyiidae, gall midges
- Ceratopogonidae, biting midges (also known as no-see-ums or punkies in North America[2] and sandflies[3] in Australia)
- Chaoboridae, phantom midges
- Chironomidae, non-biting midges (also known as muckleheads,[4] muffleheads[5] or lake flies[6] in the Great Lakes region of North America)
- Deuterophlebiidae, mountain midges
- Dixidae, meniscus midges
- Scatopsidae, dung midges
- Thaumaleidae, solitary midges
https://en.wikipedia.org/wiki/Midge
| Milk snake | |
|---|---|

| |
| Red milk snake (Lampropeltis triangulum syspila) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Reptilia |
| Order: | Squamata |
| Suborder: | Serpentes |
| Family: | Colubridae |
| Genus: | Lampropeltis |
| Species: | L. triangulum
|
| Binomial name | |
| Lampropeltis triangulum | |
| Subspecies | |
|
24 subspecies, see text | |
| Synonyms | |
| |
The milk snake or milksnake (Lampropeltis triangulum), is a species of kingsnake; 24 subspecies are currently recognized. Lampropeltis elapsoides, the scarlet kingsnake, was formerly classified as a 25th subspecies (L. t. elapsoides), but is now recognized as a distinct species.[2] The subspecies have strikingly different appearances, and many of them have their own common names. Some authorities suggest that this species could be split into several separate species.[2] They are not venomous to humans.[3][4]
https://en.wikipedia.org/wiki/Milk_snake
| African penguin | |
|---|---|

| |
| At Boulders Beach in Cape Town, South Africa | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Aves |
| Order: | Sphenisciformes |
| Family: | Spheniscidae |
| Genus: | Spheniscus |
| Species: | S. demersus
|
| Binomial name | |
| Spheniscus demersus | |

| |
| Distribution of the African penguin | |
| Synonyms | |
|
Diomedea demersa Linnaeus, 1758 | |
The African penguin (Spheniscus demersus), also known as Cape penguin or South African penguin, is a species of penguin confined to southern African waters. Like all extant penguins, it is flightless, with a streamlined body and wings stiffened and flattened into flippers for a marine habitat. Adults weigh an average of 2.2–3.5 kg (4.9–7.7 lb) and are 60–70 cm (24–28 in) tall. The species has distinctive pink patches of skin above the eyes and a black facial mask. The body's upper parts are black and sharply delineated from the white underparts, which are spotted and marked with a black band.
The African penguin is a pursuit diver and feeds primarily on fish and squid. Once extremely numerous, the African penguin is declining rapidly due to a combination of several threats and is classified as endangered. It is a charismatic species and is popular with tourists. Other vernacular names of the species include black-footed penguin and jackass penguin, due to the species' loud, donkey-like noise,[3] although several related species of South Jackass penguins produce the same sound. They can be found along the coast of South Africa and Namibia.
https://en.wikipedia.org/wiki/African_penguin
| Site of Special Scientific Interest | |
 | |
| Location | Buckinghamshire |
|---|---|
| Grid reference | TQ013842 |
| Interest | Biological |
| Area | 15.3 hectares |
| Notification | 1990 |
| Location map | Magic Map |
Black Park is a country park in Wexham, Buckinghamshire, England to the north of the A412 road. It is managed by Buckinghamshire Council, formerly County Council.[1] It has an area of 250 hectares (618 acres),[2] of which two separate areas totalling 15.7 hectares (39 acres) have been designated a biological Site of Special Scientific Interest (SSSI).[3][4] and a larger area of 66 hectares is a local nature reserve.[5][6]
https://en.wikipedia.org/wiki/Black_Park
| Capulin Volcano National Monument | |
|---|---|
 Last erupted between 55,000 to 62,000 years ago[1] | |
| Location | Raton-Clayton Volcanic Field, Union County, New Mexico, New Mexico, United States |
| Coordinates | 36°46′56″N 103°58′12″WCoordinates: 36°46′56″N 103°58′12″W |
| Area | 793 acres (321 ha)[2] |
| Elevation | 2,494 m (8,182 ft) |
| Max. elevation | 8,182 |
| Authorized | August 9, 1916 |
| Visitors | 67,442 (in 2018)[3] |
| Governing body | Department of the Interior |
| Website | Capulin Volcano National Monument |
Capulin Volcano National Monument is a U.S. National Monument located in northeastern New Mexico that protects and interprets an extinct cinder cone volcano and is part of the Raton-Clayton volcanic field. A paved road spirals gradually around the volcano and visitors can drive up to a parking lot at the rim of the extinct volcano. Hiking trails circle the rim as well as lead down into the mouth of the volcano. The monument was designated on August 9, 1916, and is administered by the National Park Service. The volcano is located 5 kilometres (3.1 mi) north of the village of Capulin.
The visitor center features exhibits about the volcano and the area's geology, natural and cultural history, and offers educational programs about volcanoes. There is also a video presentation about the volcano. The name capulin comes from a type of choke cherry, Prunus virginiana, that is native to southern North America.
Apollo 16's John Young and Charlie Duke did some of their geologic training here in May 1971. William R. Muehlberger was one of the geology instructors.[4]
https://en.wikipedia.org/wiki/Capulin_Volcano_National_Monument
 First edition hardcover | |
| Author | Victoria Foyt |
|---|---|
| Country | United States |
| Language | English |
| Series | Save the Pearls |
| Genre | Dystopian, Science fiction, Young Adult, Romance |
| Publisher | Sand Dollar Press Inc |
Publication date | January 10, 2012 |
| Media type | Print (hardback & e-book) |
| Pages | 320 (first edition, hardback) |
| ISBN | 0983650322 (first edition, hardback) |
| Followed by | Adapting Eden |
Save the Pearls: Revealing Eden is a 2012 young adult novel by American author Victoria Foyt and the first book in the Save the Pearls series. The book is set in a post-apocalyptic dystopian society and follows the titular character of Eden as she attempts to move outside of her set station in life and find a way to survive outside the norms set by society.
Book two of the series, Adapting Eden, was released in the spring of 2013.
https://en.wikipedia.org/wiki/Save_the_Pearls:_Revealing_Eden
Mountain effects
Orographic or relief snowfall is created when moist air is forced up the windward side of mountain ranges by a large-scale wind flow. The lifting of moist air up the side of a mountain range results in adiabatic cooling, and ultimately condensation and precipitation. Moisture is gradually removed from the air by this process, leaving drier and warmer air on the descending, or leeward, side.[12] The resulting enhanced snowfall,[13] along with the decrease in temperature with elevation,[14] combine to increase snow depth and seasonal persistence of snowpack in snow-prone areas.[1][15]
Mountain waves have also been found to help enhance precipitation amounts downwind of mountain ranges by enhancing the lift needed for condensation and precipitation.[16]
https://en.wikipedia.org/wiki/Snow#Mountain_effects
https://en.wikipedia.org/wiki/List_of_poems_by_Robert_Frost#Mountain_Interval_%281916%29
| Coati | |
|---|---|

| |
| White-nosed coati (Nasua narica) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Carnivora |
| Family: | Procyonidae |
| Subfamily: | Procyoninae |
| Tribe: | Procyonini |
| Subtribe: | Nasuina |
| Genera | |
| |
| Range map | |
Coatis (from Tupí),[1] also known as coatimundis (/koʊˌɑːtɪˈmʌndi/),[2][3] are members of the family Procyonidae in the genera Nasua and Nasuella. They are diurnal mammals native to South America, Central America, Mexico, and the southwestern United States. The name "coatimundi" comes from the Tupian languages of Brazil, where it means "lone coati".[4][5] Locally in Belize, the coati is known as "quash".[6]
https://en.wikipedia.org/wiki/Coati
The American Indian Wars, also known as the American Frontier Wars, and the Indian Wars, were fought by European governments and colonists in North America, and later by the United States and Canadian governments and American and Canadian settlers, against various American Indian and First Nation tribes. These conflicts occurred in North America from the time of the earliest colonial settlements in the 17th century until the early 20th century. The various wars resulted from a wide variety of factors, the most common being the desire of settlers and governments for Indian tribes' lands. The European powers and their colonies also enlisted allied Indian tribes to help them conduct warfare against each other's colonial settlements. After the American Revolution, many conflicts were local to specific states or regions and frequently involved disputes over land use; some entailed cycles of violent reprisal.
https://en.wikipedia.org/wiki/American_Indian_Wars
https://en.wikipedia.org/wiki/List_of_United_States_electric_companies
List of plants by common name
This is a list of plants organized by their common names. However, the common names of plants often vary from region to region, which is why most plant encyclopedias refer to plants using their scientific names, in other words using binomials or "Latin" names.
A
- African sheepbush – Pentzia incana
- Alder – Alnus
- Black alder – Alnus glutinosa, Ilex verticillata
- Common alder – Alnus glutinosa
- False alder – Ilex verticillata
- Gray alder – Alnus incana
- Speckled alder – Alnus incana
- White alder – Alnus incana, Alnus rhombifolia, Ilex verticillata
- Almond – Prunus dulcis
- Aloe vera – Aloe vera
- Amaranth – Amaranthus
- Foxtail amaranth – Amaranthus caudatus
- Ambrosia
- Tall ambrosia – Ambrosia trifida
- Amy root – Apocynum cannabinum
- Angel trumpet – Brugmansia suaveolens
- Apple – Malus domestica
- Apricot – Prunus armeniaca
- Arfaj – Rhanterium epapposum
- Arizona sycamore – Platanus wrighitii
- Arrowwood – Cornus florida
- Indian arrowwood – Cornus florida
- Ash – Fraxinus spp.
- Black ash – Acer negundo, Fraxinus nigra
- Blue ash – Fraxinus quadrangulata
- Cane ash – Fraxinus americana
- European ash – Fraxinus excelsior[1]
- Green ash – Fraxinus pennsylvanica lanceolata
- Maple ash – Acer negundo
- Red ash – Fraxinus pennsylvanica lanceolata
- River ash – Fraxinus pennsylvanica
- Swamp ash – Fraxinus pennsylvanica
- White ash – Fraxinus americana
- Water ash – Acer negundo, Fraxinus pennsylvanica
- Azolla – Azolla
- Carolina azolla – Azolla caroliniana
B
- Bamboo – bamboosa ardinarifolia
- Banana – mainly Musa × paradisica, but also other Musa species and hybrids
- Baobab – Adansonia
- Bay – Laurus spp. or Umbellularia spp.
- Bay laurel – Laurus nobilis (culinary)
- California bay – Umbellularia californica
- Bean – Fabaceae, specifically Phaseolus spp.
- Bearberry – Ilex decidua
- Bear corn – Veratrum viride
- Beech – Fagus
- Bindweed
- Blue bindweed – Solanum dulcamara
- Bird's nest – Daucus carota
- Bird's nest plant – Daucus carota
- Bird of paradise – Strelitzia reginae
- Birch – Betula spp.
- Black birch – Betula lenta, Betula nigra
- Bolean birch – Betula papyrifera
- Canoe birch – Betula papyrifera
- Cherry birch – Betula lenta
- European weeping birch – Betula pendula
- European white birch – Betula pendula
- Gray birch – Betula alleghaniensis
- Mahogany birch – Betula lenta
- Paper birch – Betula papyrifera
- Red birch – Betula nigra
- River birch – Betula nigra, Betula lenta
- Silver birch – Betula papyrifera
- Spice birch – Betula lenta
- Sweet birch – Betula lenta
- Water birch – Betula nigra
- Weeping birch – Betula pendula
- White birch – Betula papyrifera, Betula pendula
- Yellow birch – Betula alleghaniensis
- Bittercress – Barbarea vulgaris, Cardamine bulbosa, Cardamine hirsuta
- Hairy bittercress – Cardamine hirsuta
- Bittersweet – Solanum dulcamara
- Trailing bittersweet – Solanum dulcamara
- Bitterweed – Any plant in the genus Ambrosia, especially Ambrosia artemisiifolia, Artemisia trifida, Helenium amarum
- Blackberry – Rubus spp., Rubus pensilvanicus, Rubus occidentalis
- Hispid swamp blackberry – Rubus hispidus
- Pennsylvania blackberry – Rubus pensilvanicus
- Running swamp blackberry – Rubus hispidus
- Black cap – Rubus occidentalis
- Black-eyed Susan – Rudbeckia hirta, Rudbeckia fulgida
- Blackhaw – Viburnum prunifolium
- Blackiehead – Rudbeckia hirta
- Black-weed – Ambrosia artemisiifolia
- Blueberry – Vaccinium (Cyanococcus) spp.
- Bluebell – Hyacinthoides non-scripta
- Blue-of-the-heavens – Allium caeruleum
- Bow-wood – Maclura pomifera
- Box – Buxus
- False box – Cornus florida
- Boxelder – Acer negundo
- Boxwood – Buxus, Cornus florida
- False boxwood – Cornus florida
- Brier
- Sand brier – Solanum carolinense
- Brittlebush – Encelia farinosa
- Broadleaf – Plantago major
- Brown Betty – Rudbeckia hirta
- Brown-eyed susan – Rudbeckia hirta, Rudbeckia triloba
- Buckeye (California buckeye) – Aesculus californica
- Buckeye – Aesculus spp.
- Buffalo weed – Ambrosia trifida
- Bugle – Ajuga reptans
- Butterfly flower – Asclepias syriaca
- Butterfly weed – Asclepias tuberosa
C
- Cabbage – Brassica oleracea
- Clumpfoot cabbage – Symplocarpus foetidus
- Meadow cabbage – Symplocarpus foetidus
- Skunk cabbage – Symplocarpus foetidus, Lysichiton spp.
- Swamp cabbage – Symplocarpus foetidus
- California bay – Umbellularia californica
- California buckeye – Aesculus californica
- California sycamore – Platanus racemosa
- California walnut – Juglans californica
- Canada root – Asclepias tuberosa
- Cancer jalap – Phytolacca americana
- Carrot – Daucus carota
- Wild carrot – Daucus carota
- Carrot weed – Ambrosia artemisiifolia
- Cart track plant – Plantago major
- Catalina ironwood – Lyonothamnus floribundus ssp. floribundus
- Cedar – Cedrus spp.
- Blue Atlas cedar – Cedrus atlantica
- Deodar cedar – Cedrus deodara
- Chalk milkwort – Polygala calcarea
- Charlock – Sinapis arvensis
- Cherry – Prunus spp.
- Black cherry – Prunus serotina
- Cabinet cherry – Prunus serotina
- Rum cherry – Prunus serotina
- Whiskey cherry – Prunus serotina
- Wild cherry – Prunus avium, Prunus serotina
- Wild black cherry – Prunus serotina
- Chestnut – Castanea spp.
- Chigger flower – Asclepias tuberosa
- Chrysanthemum – Dendranthema grandiflora, Chrysanthemum morifolium
- (True) cinnamon – Cinnamomum verum
- Clove – Syzygium aromaticum
- Clover – Trifolium spp.
- Coakum – Phytolacca americana
- Coconut – Cocos nucifera
- Coffee plant – Coffea spp.
- Colic weed – Corydalis flavula
- Collard – Symplocarpus foetidus
- Columbine – Aquilegia vulgaris
- Colwort
- Hare's colwort – Sonchus oleraceus
- Comfrey – Symphytum spp.
- Coneflower
- Brilliant coneflower – Rudbeckia fulgida
- Cutleaf coneflower – Rudbeckia laciniata
- Eastern coneflower – Rudbeckia fulgida
- Green-headed Coneflower – Rudbeckia laciniata
- Orange coneflower – Rudbeckia fulgida
- Tall coneflower – Rudbeckia laciniata
- Thin-leaved Coneflower – Rudbeckia triloba
- Three-leaved Coneflower – Rudbeckia triloba
- Cornel
- Blueberry cornel – Cornus amomum
- Silky cornel – Cornus amomum
- White cornel – Cornus florida
- Cornelian tree – Cornus florida
- Corydalis – Corydalis spp.
- Fern-leaf Corydalis – Corydalis chelidoniifolia
- Golden corydalis – Corydalis aurea
- Pale corydalis – Corydalis flavula, Corydalis sempervirens
- Pink corydalis – Corydalis sempervirens
- Yellow corydalis – Corydalis lutea, Corydalis flavula
- Cotton plant – Gossypium
- Creeping yellowcress – Rorippa sylvestris
- Cress – (several genera)
- American cress – Barbarea verna
- Bank cress – Barbarea verna
- Belle Isle cress – Barbarea verna
- Bermuda cress – Barbarea verna
- Bulbous cress – Cardamine bulbosa
- Lamb's cress – Cardamine hirsuta
- Land cress – Barbarea verna, Cardamine hirsuta
- Scurvy cress – Barbarea verna
- Spring cress – Cardamine bulbosa
- Upland cress – Barbarea verna
- Crowfoot – Cardamine concatenata
- Crow's nest – Daucus carota
- Crow's toes – Cardamine concatenata
- Cucumber – Cucumis sativus
D
- Daisy
- Brown daisy – Rudbeckia hirta
- Common daisy, daisy – Bellis perennis
- Gloriosa daisy – Rudbeckia hirta
- Poorland daisy – Rudbeckia hirta
- Yellow daisy – Rudbeckia hirta
- Yellow ox-eye daisy – Rudbeckia hirta
- Deadnettle – Lamium spp.
- Henbit deadnettle – Lamium amplexicaule
- Red deadnettle – Lamium purpureum
- Spotted deadnettle – Lamium maculatum
- Desert Rose – Adenium obesum
- Devil's bite – Veratrum viride
- Devil's darning needle – Clematis virginiana
- Devil's nose – Clematis virginiana
- Devil's plague – Daucus carota
- Dewberry
- Bristly dewberry – Rubus hispidus
- Swamp dewberry – Rubus hispidus
- Dindle – Sonchus arvensis
- Dogwood – Cornus spp.
- American dogwood – Cornus florida
- Florida dogwood – Cornus florida
- Flowering dogwood – Cornus florida
- Japanese flowering dogwood – Cornus kousa
- Kousa dogwood – Cornus kousa
- Drumstick – Moringa oleifera
- Pacific dogwood – Cornus nuttallii
- Silky dogwood – Cornus amomum
- Swamp dogwood – Cornus amomum
- Duck retten – Veratrum viride
- Duscle – Solanum nigrum
- Durian – Durio zibethinus
- Durian kura kura – Durio testudinarius
- Durian munjit – Durio grandiflorus
- Durian pulu – Durio kutejensis
- Red durian – Durio dulcis
- Dye-leaves – Ilex glabra
E
- Easter orchid – Cattleya schroederae
- Earth gall – Veratrum viride
- Elderberry – Sambucus
- Elegant lupine – Lupinus elegans
- Elephant apple – Dillenia indica
- English bull's eye – Rudbeckia hirta
- Eucalyptus – Eucalyptus spp.
- Evergreen huckleberry – Vaccinium ovatum
- Extinguisher moss – Encalypta
- Eytelia – Amphipappus
F
- Fair-maid-of-France – Achillea ptarmica
- Fairymoss Azolla caroliniana
- Fellenwort – Solanum dulcamara
- Felonwood – Solanum dulcamara
- Felonwort – Solanum dulcamara
- Fennel – Foeniculum vulgare
- Ferns
- Boston fern or sword fern – Nephrolepis exaltata
- Christmas fern – Polystichum acrostichoides
- Coast polypody – Polypodium scouleri
- Kimberly queen fern – Nephrolepis obliterata
- Korean rock fern – Polystichum tsus-simense
- Mosquito fern – Azolla caroliniana
- Sword ferns – Polystichum spp.
- Water fern – Azolla caroliniana
- Western sword fern – Polystichum munitum
- Feverbush – Ilex verticillata
- Feverfew – Tanacetum parthenium
- Field forget-me-not – Myosotis arvensis
- Fig – Ficus spp.
- Common fig – Ficus carica
- Flax
- European flax – Linum usitatissimum
- New Zealand flax – Phormium tenax, Phormium colensoi
- Fluxroot – Asclepias tuberosa
- Foxglove – Digitalis purpurea
- Fumewort
- Yellow fumewort – Corydalis flavula
G
- Gallberry – Ilex glabra
- Garget – Phytolacca americana
- Garlic
- Golden garlic – Allium moly
- Wild garlic – Allium canadense
- Garlic mustard – Alliaria petiolata
- Garlic root – Alliaria petiolata
- Germander speedwell – Veronica chamaedrys
- Gilliflower
- Dame's gilliflower – Hesperis matronalis
- Night scented gilliflower – Hesperis matronalis
- Queen's gilliflower – Hesperis matronalis
- Rogue's gilliflower – Hesperis matronalis
- Winter gilliflower – Hesperis matronalis
- Golden buttons – Tanacetum vulgare
- Golden chain – Laburnum
- Goldenglow – Rudbeckia laciniata
- Golden Jerusalem – Rudbeckia hirta
- Gordaldo – Achillea millefolium
- Goose tongue – Achillea ptarmica
- Grapefruit – Citrus × paradisi
- Grapevine – Vitis
- Grass - Poaceae
- Bermuda grass - Cynodon dactylon
- Green berry nightshade – Solanum opacum
- Groundberry – Rubus hispidus
- Bristly groundberry – Rubus hispidus
- Gutweed – Sonchus arvensis
H
- Haldi – Curcuma domestica
- Harlequin
- Rock harlequin – Corydalis sempervirens
- Yellow harlequin – Corydalis flavula
- Hay fever weed – Ambrosia artemisiifolia, Artemisia trifida
- Healing blade – Plantago major
- Hedge plant – Maclura pomifera
- Hellebore – Helleborus
- American white hellebore – Veratrum viride
- Big hellebore – Veratrum viride
- Black hellebore – Veratrum nigrum
- European white hellebore – Veratrum album
- False hellebore – Veratrum album, Veratrum viride
- Swamp hellebore – Veratrum viride
- White hellebore – Veratrum album, Veratrum viride
- Hemp – Cannabis spp., specifically Cannabis sativa
- Hemp dogbane – Apocynum cannabinum
- Hen plant – Plantago major
- Herb barbara – Barbarea vulgaris
- Hogweed – Ambrosia artemisiifolia
- Holly – Ilex spp.
- Deciduous holly – Ilex decidua, Ilex verticillata
- European holly – Ilex aquifolium
- Inkberry holly – Ilex glabra
- Meadow holly – Ilex decidua
- Swamp holly – Ilex decidua
- Winterberry holly – Ilex verticillata
- Honesty – Lunaria annua
- Horse cane – Ambrosia trifida
- Horseradish – Armoracia rusticana
- Hound's berry – Solanum nigrum
- Huckleberry – Vaccinium spp.
- Evergreen huckleberry – Vaccinium ovatum
- Trailing red huckleberry – Vaccinium parvifolium
- Houseleek – Sempervivum tectorum
- Hydrangea – Hydrangea macrophylla
I
- Indian hemp – various genera
- Indian hemp – Apocynum cannabinum, Cannabis indica
- White Indian hemp – Asclepias incarnata
- Indian paintbrush – Castilleja, Castilleja mutis, Asclepias tuberosa
- Indian posy – Asclepias tuberosa
- Inkberry – Ilex glabra, Phytolacca americana
- Ironwood – Acacia estrophiolata
- Isle of Man cabbage – Coincya monensis
- Itchweed – Veratrum viride
- Ivy – Hedera spp.
J
- Jack-by-the-hedge – Alliaria petiolata
- Jack-in-the-bush – Alliaria petiolata
- Jasmine – Jasminum officinale
- Jewel orchid – Ludisia discolor
- Jointed rush – Juncus kraussii
- Jugflower – Adenanthos obovatus
- Juneberry – Amelanchier canadensis
- Juniper – Various species in the genus Juniperus
K
- Keek – Rorippa sylvestris
- King fern – Ptisana salicina
- Kinnikinnik – Cornus amomum
- Kittentail – Veronica bullii
- Knotweed (Japanese) – Reynoutria japonica (syn. Fallopia japonica)
- Kousa – Cornus kousa
- Kudzu – Pueraria montana
- Kumarahou – Pomaderris kumeraho
L
- Laceflower – Daucus carota
- Lace fern – Asparagus setaceus
- Lady's mantle – Alchemilla mollis
- Lady's smock – Cardamine pratensis
- Lamb's foot – Plantago major
- Latanier palm – Phoenicophorium
- Laurel magnolia – Magnolia splendens
- Lavender – Lavandula
- Leek – Allium
- Lemon – Citrus × limon
- Leopard lily – Lilium catesbaei
- Lily of the Nile – Agapanthus praecox
- Lettuce – Lactuca sativa
- Lily leek – Allium moly
- Lilac
- Summer lilac – Hesperis matronalis
- Little sunflower – Helianthella
- Lone fleabane – Erigeron cavernensis
- Love-lies-bleeding – Amaranthus caudatus
- Love vine – Clematis virginiana
- Lupin – Lupinus
M
- Magnolia – Magnolia
- Bracken's Brown Beauty magnolia – Magnolia grandiflora
- Galaxy magnolia – Magnolia x
- Jane magnolia – Magnolia x
- Kay Parris magnolia – Magnolia grandiflora
- Little Gem magnolia – Magnolia grandiflora
- Moonglow magnolia – Magnolia virginiana
- Royal Star magnolia – Magnolia stellata
- Serendipity Ruby magnolia – Magnolia figo
- Southern magnolia – Magnolia grandiflora
- Stellar Ruby magnolia – Magnolia figo
- Sweetbay magnolia – Magnolia virginiana
- Waterlilly Star magnolia – Magnolia stellata
- Maize – Zea mays
- Mango – Mangifera indica
- Maple – Acer
- Ash-leaved maple – Acer negundo
- Black maple – Acer nigrum
- Creek maple – Acer saccharinum
- Cutleaf maple – Acer negundo
- Maple ash – Acer negundo
- Moose maple – Acer pensylvanicum
- Red river maple – Acer negundo
- River maple – Acer saccharinum
- Silver maple – Acer saccharinum
- Silverleaf maple – Acer saccharinum
- Soft maple – Acer saccharinum
- Striped maple – Acer pensylvanicum
- Sugar maple – Acer saccharum (main use), Acer barbatum, Acer leucoderme,
- Swamp maple – Acer saccharinum
- Water maple – Acer saccharinum
- White maple – Acer saccharinum
- Mesquite – Prosopis
- Honey mesquite – Prosopis glandulosa
- Screwbean mesquite – Prosopis pubescens
- Milfoil – Achillea millefolium
- Milkweed – Asclepias, Sonchus oleraceus
- Blunt-leaved Milkweed – Asclepias amplexicaulis
- Common milkweed – Asclepias syriaca
- Horsetail milkweed – Asclepias verticillata
- Orange milkweed – Asclepias tuberosa
- Swamp milkweed – Asclepias incarnata
- Rose milkweed – Asclepias incarnata
- Whorled milkweed – Asclepias verticillata
- Yellow milkweed – Asclepias tuberosa
- Milky tassel – Sonchus oleraceus
- Moosewood – Acer pensylvanicum
- Petty morel – Solanum nigrum
- Morelle verte – Solanum opacum
- Mosquito plant – Azolla caroliniana
- Mother-of-the-evening – Hesperis matronalis
- Mountain mahogany – Betula lenta
- Mulberry – Morus
- Red mulberry – Morus rubra
- White mulberry – Morus alba
N
- Native fuchsia – Epacris longiflora
- Necklace fern – Asplenium flabellifolium
- Neem – Azadirachta indica
- Nettle – Urtica dioica
- Bull nettle – Solanum carolinense
- Carolina horse nettle – Solanum carolinense
- Horse nettle – Solanum carolinense
- Night-blooming cactus – Hylocereus
- Nightshade
- American nightshade – Phytolacca americana, Solanum americanum
- Bitter nightshade – Solanum dulcamara
- Black nightshade – Solanum nigrum, Solanum americanum
- Climbing nightshade – Solanum dulcamara
- Deadly nightshade – Atropa belladonna
- Garden nightshade – Solanum nigrum
- Trailing nightshade – Solanum dulcamara
- Trailing violet nightshade – Solanum dulcamara
- Woody nightshade – Solanum dulcamara
- Nodding wakerobin – Trillium cernuum
- Northern moonwort – Botrychium boreale
- Nosebleed – Achillea millefolium
O
- Oak tree – Quercus
- Algerian Oak – Quercus canariensis
- Blue oak – Quercus douglasii
- Bur oak – Quercus macrocarpa
- California Black Oak Quercus kelloggii
- Canyon Live Oak Quercus chrysolepis
- Champion oak – Quercus rubra
- Coast live oak – Quercus agrifolia
- Cork oak – Quercus suber
- Dyer's oak – Quercus velutina
- Eastern black oak – Quercus velutina
- English oak – Quercus robur
- Island oak – Quercus tomentella
- Mirbeck's oak – Quercus canariensis
- Mossycup white oak – Quercus macrocarpa
- Northern red oak – Quercus rubra
- Pedunculate oak – Quercus robur
- Pin oak – Quercus palustris
- Red oak – Quercus rubra, Quercus coccinea
- Scarlet oak – Quercus coccinea
- Scrub oak – Quercus macrocarpa
- Sessile oak – Quercus petraea
- Spanish oak – Quercus coccinea, Quercus rubra
- Spotted oak – Quercus velutina
- Swamp oak – Quercus palustris, Quercus bicolor
- Swamp Spanish oak – Quercus palustris
- Swamp white oak – Quercus bicolor
- Valley oak – Quercus lobata
- White oak – Quercus alba
- Yellowbark oak – Quercus velutina
- Obedient Plant – Physostegia virginiana
- Olive – Olea europaea
- Onion – Allium
- Common onion – Allium cepa
- Giant onion – Allium giganteum
- Nodding onion – Allium cernuum
- Tree onion – Allium canadense
- Wild onion – Allium canadense
- Orange –
- Osage orange – Maclura pomifera
- Sweet orange – Citrus × sinensis
- Wild orange – Maclura pomifera
- Orange-root – Asclepias tuberosa
- Osage – Maclura pomifera
- Osier – Salix; (in North America) Cornus
- Red osier – Cornus amomum
P
- Parsley – Petroselinum crispum
- Parsnip – Pastinaca sativa, Daucus carota
- Pawpaw - Asimina triloba
- Pea – Pisum sativum
- Peach – Prunus persica
- Peanut – Arachis hypogaea
- Pear – Pyrus
- Pellitory
- Bastard pellitory – Achillea ptarmica
- European pellitory – Achillea ptarmica
- Wild pellitory – Achillea ptarmica
- Penny hedge – Alliaria petiolata
- Pepper root – Cardamine concatenata
- Petty spurge – Euphorbia peplus
- Pigeon berry – Phytolacca americana
- Pine – Pinus
- Loblolly Pine – Pinus taeda
- Pineapple – Ananas comosus
- Pistachio – Pistacia vera
- Plane (European sycamore) – Platanus acerifolia
- Plantain
- Broadleaf plantain – Plantago major
- Common plantain – Plantago major
- Dooryard plantain – Plantago major
- Greater plantain – Plantago major
- Roundleaf plantain – Plantago major
- Wayside plantain – Plantago major
- Pleurisy root – Asclepias tuberosa
- Poached egg plant – Limnanthes douglasii
- Pocan bush – Phytolacca americana
- Poison ivy – Toxicodendron radicans
- Poisonberry – Solanum dulcamara
- Poisonflower – Solanum dulcamara
- Poke – Phytolacca americana
- Indian poke – Veratrum viride
- Pokeroot – Phytolacca americana
- Pokeweed – Phytolacca americana
- Polkweed – Symplocarpus foetidus
- Polecat weed – Symplocarpus foetidus
- Poor Annie – Veratrum viride
- Poor man's mustard – Alliaria petiolata
- Poplar – Populus
- Poppy – Papaveraceae
- Possumhaw – Ilex decidua
- Potato – Solanum tuberosum
- Primrose – Primula vulgaris
Q
- Queen Anne's lace – Daucus carota, Anthriscus sylvestris
- Quercitron – Quercus velutina
R
- Radical weed – Solanum carolinense
- Ragweed – Ambrosia
- Common ragweed – Ambrosia artemisiifolia
- Giant ragweed – Ambrosia trifida
- Great ragweed – Ambrosia trifida
- Ragwort – Senecio
- Common ragwort – Senecio jacobaea
- Hoary ragwort – Senecio erucifolius
- Marsh ragwort – Senecio aquaticus
- Oxford ragwort – Senecio squalidus
- Silver ragwort – Senecio cineraria
- Rantipole – Daucus carota
- Rapeseed – Brassica napus
- Raspberry – Rubus (Idaeobatus) spp.
- Black raspberry – Rubus occidentalis
- Purple raspberry – Rubus occidentalis
- Redbrush – Cornus amomum
- Redbud – Cercis spp.
- Eastern redbud – Cercis canadensis
- Western redbud – Cercis occidentalis
- Judas-tree – Cercis siliquastrum
- Red ink plant – Phytolacca americana
- Redweed – Phytolacca americana
- Rheumatism root – Apocynum cannabinum
- Rhubarb – Rheum rhabarbarum
- Ribwort – Plantago major
- Rice
- Asian rice – Oryza sativa
- African rice – Oryza glaberrima
- Roadweed – Plantago major
- Rocket – (several genera)
- Dame's rocket – Hesperis matronalis
- Sweet rocket – Hesperis matronalis
- Winter rocket – Barbarea vulgaris
- Yellow rocket – Barbarea vulgaris
- Rocketcress – Barbarea vulgaris
- Rose – Rosa
- Baby rose – Rosa multiflora
- Dwarf wild rose – Rosa virginiana
- Low rose – Rosa virginiana
- Multiflora rose – Rosa multiflora
- Prairie rose – Rosa virginiana
- Rambler rose – Rosa multiflora
- Wild rose – Rosa virginiana
- Rosemary – Rosmarinus officinalis
- Rye – Secale cereale
S
- Saffron crocus – Crocus sativus
- Sanguinary – Achillea millefolium
- Saskatoon – Amelanchier alnifolia
- Sauce-alone – Alliaria petiolata
- Scarlet berry – Solanum dulcamara
- Scoke – Phytolacca americana
- Scotch cap – Rubus occidentalis
- Scrambled eggs – Corydalis aurea
- Scurvy grass – Barbarea verna
- Serviceberry – Amelanchier
- Common serviceberry – Amelanchier arborea
- Downy serviceberry – Amelanchier arborea
- Shadblow serviceberry – Amelanchier canadensis
- Shadblow – Amelanchier canadensis
- Shadbush – Amelanchier canadensis
- Shrubby mayweed – Oncosiphon suffruticosum
- Silkweed – Asclepias syriaca
- Swamp silkweed – Asclepias incarnata
- Virginia silkweed – Asclepias syriaca
- Skunkweed – Symplocarpus foetidus
- Snakeberry – Solanum dulcamara
- Snowdrop – Galanthus
- Sorrel – Oxalis
- Redwood sorrel – Oxalis oregana
- Speedwell
- Corn speedwell – Veronica arvensis
- Wall speedwell – Veronica arvensis
- Spikenard – Nardostachys jatamansi
- Spoolwood – Betula papyrifera
- Squaw bush – Cornus amomum
- Stammerwort – Ambrosia artemisiifolia
- Star-of-Persia – Allium cristophii
- Stickweed – Ambrosia artemisiifolia
- Strawberry – Fragaria × ananassa
- Strawberry tree – Arbutus unedo
- Strawberry tree 'Marina' – Madrone – Arbutus 'Marina'
- Sugarcane – Saccharum
- Swallow-wort
- Orange swallow-wort – Asclepias tuberosa
- Silky swallow-wort – Asclepias syriaca
- Sneezeweed – Achillea ptarmica
- Sneezewort – Achillea ptarmica
- Sunflower – Helianthus annuus
- Sugarplum – Amelanchier canadensis
- Soldier's woundwort – Achillea millefolium
- Stag bush – Viburnum prunifolium
- Swallow-wort
- Orange swallow-wort – Asclepias tuberosa
- Silky swallow-wort – Asclepias syriaca
- Sweet potato – Ipomoea batatas
- Sweet potato vine – Ipomoea batatas
- Swinies – Sonchus oleraceus
- Sycamore – Platanus spp.
- Sycamore (California) – Platanus racemosa
- Sycamore (Arizona) – Platanus wrighitii
- Sycamore (American) – Platanus occidentalis
- Sundari (Bangladesh) – Heritiera fomes
- Gewa (Bangladesh) – Excoecaria agallocha
T
- Tansy
- Common tansy – Tanacetum vulgare
- White tansy – Achillea ptarmica
- Wild tansy – Ambrosia artemisiifolia
- Tea – Camellia sinensis
- Appalachian tea – Ilex glabra
- Thimbleberry – Rubus occidentalis
- Thimbleweed – Rudbeckia laciniata
- Thousand-leaf – Achillea millefolium
- Thousand-seal – Achillea millefolium
- Tassel weed – Ambrosia artemisiifolia
- Thistle – (Several genera)
- Annual sow thistle – Sonchus oleraceus
- California thistle – Cirsium arvense
- Canada thistle – Cirsium arvense
- Corn thistle – Cirsium arvense
- Corn sow thistle – Sonchus arvensis
- Creeping thistle – Cirsium arvense
- Cursed thistle – Cirsium arvense
- Field sow thistle – Sonchus arvensis
- Green thistle – Cirsium arvense
- Hard thistle – Cirsium arvense
- Hare's thistle – Sonchus oleraceus
- Milk thistle – Sonchus oleraceus
- Nodding thistle – Carduus nutans L.
- Perennial thistle – Cirsium arvense
- Prickly thistle – Cirsium arvense
- Sharp-fringed sow Thistle – Sonchus asper
- Small-flowered Thistle – Cirsium arvense
- Spiny sow thistle – Sonchus asper
- Spiny-leaved sow Thistle – Sonchus asper
- Swine thistle – Sonchus arvensis
- Tree sow thistle – Sonchus arvensis
- Way thistle – Cirsium arvense
- Thyme – Thymus, specifically Thymus vulgaris
- Tickleweed – Veratrum viride
- Tobacco plant – Nicotiana
- Tomato – Solanum lycopersicum
- Toothwort – Cardamine concatenata
- Cutleaf toothwort – Cardamine concatenata
- Purple-flowered Toothwort – Cardamine concatenata
- Touch-me-not – Impatiens capensis, Impatiens pallida, Mimosa pudica, Cardamine hirsuta
- Traveller's joy – Clematis virginiana
- Tread-softly – Solanum carolinense
- Tree tobacco – Nicotiana glauca
- Trillium – Trillium spp.
- Western trillium – Trillium ovatum
- Western wake robin – Trillium ovatum
- White trillium – Trillium grandiflorum
- Western trillium – Trillium ovatum
- Tuber-root – Asclepias tuberosa
- Tulip – Tulipa
- Tulsi – Ocimum santalum
U
- Umbrella palm – Hedyscepe canterburyana
- Umbrella papyrus – Cyperus alternifolius
V
- Vanilla orchid – Vanilla
- Varnish tree – Koelreuteria paniculata
- Velvet bean – Mucuna pruriens
- Viburnum – Viburnum
- Blackhaw viburnum – Viburnum prunifolium
- Leatherleaf viburnum – Viburnum rhytidophyllum
- Violet – (several genera)
- Viola species
- African violet – Streptocarpus sect. Saintpaulia species
- Damask violet – Hesperis matronalis
- Dame's violet – Hesperis matronalis
- Dog's-tooth-violet or dogtooth violet – Erythronium dens-canis
- Violet bloom – Solanum dulcamara
- Viper's grass – Scorzonera hispanica
- Virgin's bower – Clematis virginiana
- Virginia virgin's bower – Clematis virginiana
- Voodoo lily – Dracunculus vulgaris
W
- Walnut – Juglans sp.
- Wattle – Acacia
- Mount Connor wattle – Acacia ammobia
- Waybread – Plantago major
- Weed (Marijuana) — Cannabis Sativa, Cannabis Indica, Cannabis Ruderalis
- Western redbud – Cercis occidentalis
- Wheat – Triticum spp.
- White man's foot – Plantago major
- White-root – Asclepias tuberosa
- Wild cotton – Apocynum cannabinum, Asclepias syriaca
- Wild honeysuckle – Lambertia
- Prickly honeysuckle – Lambertia echinata
- Heath-leaved honeysuckle – Lambertia ericifolia
- Fairall's honeysuckle – Lambertia fairallii
- Mountain devil – Lambertia formosa
- Honey flower – Lambertia formosa
- Holly-leaved honeysuckle – Lambertia ilicifolia
- Chittick – Lambertia inermis
- Many-flowered honeysuckle – Lambertia multiflora
- Round-leaf honeysuckle – Lambertia orbifolia
- Green honeysuckle – Lambertia rariflora
- Wild hops – Clematis virginiana
- Willow – Salix
- Coyote willow – Salix exigua
- Goodding willow – Salix gooddingii
- Red willow – Cornus amomum
- Rose willow – Cornus amomum
- Windroot – Asclepias tuberosa
- Wineberry – Rubus phoenicolasius
- Winterberry – Ilex verticillata
- American winterberry – Ilex verticillata
- Evergreen winterberry – Ilex glabra
- Virginia winterberry – Ilex verticillata
- Wintercress – Barbarea vulgaris
- Early wintercress – Barbarea verna
- Woodbine – Clematis virginiana
- Woolly morning glory – Argyreia nervosa
- Wormwood
- Roman wormwood – Ambrosia artemisiifolia, Corydalis sempervirens
- Wound rocket – Barbarea vulgaris
X
Y
- Yarrow – Achillea
- Common yarrow – Achillea millefolium
- Fernleaf yarrow – Achillea filipendulina
- Sneezewort yarrow – Achillea ptarmica
- Woolly yarrow – Achillea tomentosa
- Yellow fieldcress – Rorippa sylvestris
- Yellowwood – Cladrastis lutea, Maclura pomifera
- Yellow coneflower – Echinacea paradoxa
- Yam – Dioscorea; (in North America) Ipomoea batatas
- Yunnan camellia – Camellia yunnanensis
Z
- Zebrawood – Brachystegia spiciformis
- Zedoary – Curcuma zedoaria
See also
References
- "White Ash Burns With Fall Splendor". The Spruce. Retrieved 2018-09-24.
https://en.wikipedia.org/wiki/List_of_plants_by_common_name
| Black Hills Expedition (1874) | |||||
|---|---|---|---|---|---|
| Part of the Sioux Wars | |||||
| |||||
| Belligerents | |||||
|
| |||||
| Commanders and leaders | |||||
|
| |||||
| Units involved | |||||
| 7th United States Cavalry Regiment | |||||
| Strength | |||||
| ~1,200 Soldiers and Civilians | |||||
| Casualties and losses | |||||
| 1 killed | |||||
The Black Hills Expedition was a United States Army expedition in 1874 led by Lieutenant Colonel George Armstrong Custer that set out on July 2, 1874, from modern day Bismarck, North Dakota, which was then Fort Abraham Lincoln in the Dakota Territory, with orders to travel to the previously uncharted Black Hills of South Dakota. Its mission was to look for suitable locations for a fort, find a route to the southwest, and to investigate the possibility of gold mining.[1] Custer and his unit, the 7th Cavalry, arrived in the Black Hills on July 22, 1874, with orders to return by August 30. The expedition set up a camp at the site of the future town of Custer; while Custer and the military units searched for a suitable location for a fort, civilians searched for gold, and it is disputed whether or not any substantial amount was found. Nonetheless, this prompted a mass gold rush which in turn antagonised the Sioux Indians who had been promised protection of their sacred land through Treaties made by the US government,[2] and who were later to kill Custer at the Battle of the Little Big Horn in the Great Sioux War of 1876–1877 between themselves and the United States.[1]
The entire expedition was photographed by William H. Illingworth, an English photographer who accompanied Custer after selection by the then-Captain William Ludlow. Ludlow, the engineer for the expedition, financed Illingworth's photography and paid him $30 per month to provide photographic plates for the US Army, of which he made 70 in all.
https://en.wikipedia.org/wiki/Black_Hills_Expedition
| Cat Temporal range: 9,500 years ago – present
| |
|---|---|
Various types of cats | |
Domesticated
| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Carnivora |
| Suborder: | Feliformia |
| Family: | Felidae |
| Subfamily: | Felinae |
| Genus: | Felis |
| Species: | F. catus[1]
|
| Binomial name | |
| Felis catus[1] | |
| Synonyms | |
The cat (Felis catus) is a domestic species of small carnivorous mammal.[1][2] It is the only domesticated species in the family Felidae and is commonly referred to as the domestic cat or house cat to distinguish it from the wild members of the family.[4] Cats are commonly kept as house pets but can also be farm cats or feral cats; the feral cat ranges freely and avoids human contact.[5] Domestic cats are valued by humans for companionship and their ability to kill small rodents. About 60 cat breeds are recognized by various cat registries.[6]
https://en.wikipedia.org/wiki/Cat
Nevada State Route 375
| Extraterrestrial Highway Warm Springs Road | ||||
 | ||||
| Route information | ||||
| Maintained by NDOT | ||||
| Length | 98.443 mi[1] (158.429 km) | |||
| Existed | 1976–present | |||
| History | SR 25 by 1933; became SR 375 in 1976; highway named in 1996. | |||
| Major junctions | ||||
| South end | ||||
| North end | ||||
| Location | ||||
| Country | United States | |||
| State | Nevada | |||
| Counties | Nye, Lincoln | |||
| Highway system | ||||
| ||||
| ||||
State Route 375 (SR 375) is a 98.414-mile (158.382 km) state highway in Nye and Lincoln counties in south-central Nevada, United States. The highway stretches from State Route 318 at Crystal Springs northwest to U.S. Route 6 (US 6) at Warm Springs. The route travels through mostly unoccupied desert terrain, with much of its alignment paralleling the northern edges of the Nellis Air Force Range. The road originally traversed through what is now the northern reaches of the air force range in the 1930s, when it was previously designated State Route 25A and later part of State Route 25.
The top-secret Area 51 government base is near SR 375, and many travelers have reported UFO observations and other strange alien activity along this road. Such stories prompted the state to officially designate the route as the Extraterrestrial Highway in 1996. The small town of Rachel, located near the midpoint of the highway, caters to tourists, geocachers, and UFO seekers with alien-themed businesses. Although the area receives some tourism due to alleged extraterrestrial activity, SR 375 remains a lightly traveled route.
Route description
State Route 375 begins at a "Y" junction with State Route 318 at Crystal Springs, a ghost town in the northern end of the Pahranagat Valley in the center of Lincoln County. The site, which is little more than the junction and a few trees, functions as a rest area.[2] From Crystal Springs, the highway curves southwest to pass between the Pahrangat and Mount Irish ranges to ascend 5,592-foot (1,704 m) Hancock Summit.[2]
Descending the summit, SR 375 nears the border of the Nellis Air Force Range. As the highway heads northwest through Tikaboo Valley, it meets Mail Box Road. It used to be marked by a single postal drop known as the "black mailbox": the dirt access road leads to the restricted lands surrounding Area 51. The original mailbox was a normal black mailbox, however due to people constantly sifting through it, it was replaced with a white one that was much more secure. The name "Black Mailbox" still stuck. It was commonly used as a gathering place for UFO seekers,[3] and two to three UFO sightings per week allegedly occur in the area.[4] The mailbox was removed by its owner, Steve Medlin, due to continued vandalism.[5] SR 375 continues heading northwest from the mailbox, climbing in elevation again to reach the top of Coyote Summit at 5,591 feet (1,704 m).[2]
West of the summit, the Extraterrestrial Highway descends into the Sand Spring Valley and the community of Rachel becomes visible. The small town of about 50 residents is little more than homes and a few businesses. The Little A'Le'Inn (pronounced "alien") is the focal point of the town, providing a small motel, an alien-themed restaurant/bar, and extraterrestrial souvenirs.[4][6] The civilian-run Area 51 Research Center, based out of a yellow house trailer[7] and documenting paranormal activity in the area, closed in 2001.[4][8] As of early 2021, there is now a gas station and general store open in Rachel, with a campground and RV hookup planned to open by spring of 2022.[citation needed]
Leaving Rachel, SR 375 continues northwest to enter Nye County. The route climbs out of Sand Spring Valley and heads over the 5,935-foot (1,809 m) Queen City Summit, the highest point on the highway.[2] After passing the summit, the route descends into the southern end of Railroad Valley, curving nearly due north for several miles as it follows the base of Reveille Range. As the mountains subside, the road turns westward again to head to its northern terminus at the junction of US 6 at Warm Springs.[2]
History
Route development
An unimproved road approximating the present alignment of State Route 375 came into existence by 1932. This route, christened State Route 25A, connected Crystal Springs to State Route 4 (now US 6) just east of Tonopah.[9] By 1933, SR 25A had been renumbered to become a new western segment of State Route 25.[10] The route underwent periodic realignments over the next few years, but the highway's terminal junctions remained mostly unchanged.
In 1942, SR 25 appeared to have a significant gap in its route. State maps from the time show a large area within Nye and Lincoln Counties where all roads had been removed.[11] The route existed in one piece again by 1946, although it had been realigned northward and shortened to 111 miles (179 km).[12] A sizable portion of SR 25 passing through the Tonopah U.S. Army Air Force Bombing Range (now the Nellis Air Force Range and Nevada Test Site) was restricted from public travel by 1950, the restricted section being approximately the same area that was removed in 1942.[11][13] To avoid the restricted area of the testing range, the west end of SR 25 was realigned by 1957. The highway connected to US 6 at Warm Springs about 37 miles (60 km) east of the previous terminus, heading north around the Reveille Range instead of climbing the Kawich Range within the bombing area.[14]
With the 1957 realignment, the routing of SR 25 attained its final form. The entire highway was paved by the following year.[15] SR 25 remained unchanged until the 1976 renumbering of Nevada's state highways, through which the western section of SR 25 became the new State Route 375.[16] The new route number was first seen on the 1978 edition of the official state highway map.[17]
Naming the highway
In 1989, an engineer named Bob Lazar claimed to have worked on alien spaceships and to have viewed saucer test flights in Tikaboo Valley, telling his story to a Las Vegas television station which was subsequently broadcast as an exclusive report.[3] By the 1990s, stories of the top-secret U.S. government base at Area 51 had become mainstream, and many books and personal accounts had been published regarding extraterrestrial spacecraft and alien activity in the region surrounding Groom Lake. Rachel, being the closest settlement to the restricted facility, attracted people in search of UFOs and alien life.[18] To capitalize on the purported paranormal activity along the route, the Nevada Commission on Tourism sought to rename the highway. State officials drew inspiration from the alien legends and dubbed SR 375 the Extraterrestrial Highway in February 1996.[19] The tourism commission hoped that the renaming would "draw travelers to the austere and remote reaches of south-central Nevada, where old atomic bomb test sites, secret Defense Department airstrips and huge, sequestered tracts of military land create a marketable mystique".[6]
News of the highway's renaming reached Twentieth Century Fox. The studio used the opportunity to promote the release of the film Independence Day, whose plot involves an alien invasion of Earth and the secret facility at Area 51. A public dedication ceremony for the Extraterrestrial Highway was held in Rachel in April 1996.[20] State dignitaries at the ceremony were joined by studio executives and Independence Day stars Jeff Goldblum, Robert Loggia, Bill Pullman, and Brent Spiner.[20][21] Nevada Governor Bob Miller presided over the ceremony, speaking with humorous space references and unveiling special "Extraterrestrial Highway 375" and "Speed Limit Warp 7" signs for the highway.[21] The event concluded with several guests placing items related to Nevada and the film into a time capsule commemorating the occasion.[20][21]
To promote the Extraterrestrial Highway after its renaming, the tourism commission launched "The ET Experience" in July 1996. Tourists could contact the Nevada Commission on Tourism to receive a traveler's kit containing information about the highway, nearby cultural attractions, and area services. Visitors that patronized businesses in Rachel and central Nevada and submitted an account of their journey received Extraterrestrial Highway memorabilia. Stories from travelers were also published in a newsletter available to those that had completed the experience.[19] Despite tourism generated by people searching for signs of alien life, only "an average of about 200 cars drive some portion of the Extraterrestrial Highway every day, making it one of the state’s least traveled routes."[6]
In keeping with the supposed alien links of the area, in 2006, KFC established what was said to be the first corporate logo visible from space, made from 87,500 square feet (8,130 m2) of tiles and sited just off the Extraterrestrial Highway in Rachel.[22][23] The logo has since been removed.[24]
Major intersections
- Note: Mileposts in Nevada reset at county lines. The start and end mileposts for each county are given in the county column.
| County | Location | mi[1] | km | Destinations | Notes |
|---|---|---|---|---|---|
| Lincoln 0.00-49.102 | Crystal Springs | 0.000 | 0.000 | Southern terminus | |
| Nye 0.00-49.341 | Warm Springs | 98.443 | 158.429 | Northern terminus | |
| 1.000 mi = 1.609 km; 1.000 km = 0.621 mi | |||||
See also
- List of state highways in Nevada
- State Route 319, the eastern segment of former State Route 25
References
- Jennings, Ken (June 9, 2014). "The Fast-Food Advertisement That Was Visible From Space". Conde Nast Traveler. Retrieved June 10, 2019.
External links
Route map:
- Extraterrestrial Highway Home Page, includes photos and many useful links
- Extraterrestrial Highway at Vegas.com, includes short video preview
- The Extraterrestrial Highway at Roadtrip America
- Nevada 375/Extraterrestrial Highway at AARoads
https://en.wikipedia.org/wiki/Nevada_State_Route_375
https://en.wikipedia.org/wiki/Great_Salt_Lake
https://en.wikipedia.org/wiki/Active_volcano
https://en.wikipedia.org/wiki/Army_Combat_Uniform
| Arabia Mountain | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 View from the summit of Arabia Mountain | |||||||||||||||||
| Highest point | |||||||||||||||||
| Elevation | 955 ft (291 m) | ||||||||||||||||
| Prominence | 172 ft (52 m) | ||||||||||||||||
| Coordinates | 33°39′54″N 84°7′6″W | ||||||||||||||||
| Geography | |||||||||||||||||
| Location | DeKalb County, Georgia | ||||||||||||||||
| Climbing | |||||||||||||||||
| First ascent | unknown | ||||||||||||||||
| Easiest route | Hike | ||||||||||||||||
| |||||||||||||||||
Arabia Mountain, a part of Arabia Mountain National Heritage Area, is the northern of two peaks in the Davidson-Arabia Mountain Nature Preserve, in DeKalb County, Georgia, United States. A low saddle separates it from Bradley Mountain, several hundred feet to its south. The two form a monadnock. The peak is 955 feet (290 m) above sea level, rising 172 feet (52 m) above Arabia Lake reservoir. Bradley Mountain is closer to the visitor trails than Arabia Mountain and is often misidentified by visitors as Arabia Mountain.
The mountain is also in a namesake National Heritage Area[1] in the U.S. state of Georgia that encompasses natural, cultural, and historical elements to form a cohesive, nationally significant landscape.[2] The area is due east of Atlanta and spans 40,000 acres (16,000 ha) reaching from the historic commercial center of Lithonia to the Monastery of the Holy Spirit in Conyers, including a number of sites in between, including Panola Mountain State Park, Davidson-Arabia Mountain Nature Preserve, the historic Flat Rock Community with the Flat Rock Archives, and more. The National Heritage Area was established in 2006 and is coordinated by the Arabia Mountain National Heritage Area Alliance, which includes board members, representatives from the community and local organizations, and staff.
Additionally, Davidson-Arabia Mountain Nature Preserve includes 2,550 acres in DeKalb County, Georgia with a multi-use bike path, hiking trails, and lakes for fishing. The park features large exposed granite formations, wetlands, pine forest, oak forest, streams habitat and two lakes. Plant species include the rare red diamorpha in the winter and yellow daises in the fall. The area included rock quarries and abandoned structures from the mining operations.[3]
https://en.wikipedia.org/wiki/Arabia_Mountain
Georgia O'Keeffe | |
|---|---|
 O'Keeffe in 1918, photograph by Alfred Stieglitz | |
| Born | Georgia Totto O'Keeffe November 15, 1887 Sun Prairie, Wisconsin, U.S. |
| Died | March 6, 1986 (aged 98) Santa Fe, New Mexico, U.S. |
| Education | School of the Art Institute of Chicago Columbia College Teachers College, Columbia University University of Virginia Art Students League of New York |
| Known for | Painting |
| Movement | American modernism, Precisionism |
| Spouse | |
| Family | Ida O'Keeffe (sister) |
| Awards | National Medal of Arts (1985) Presidential Medal of Freedom (1977) Edward MacDowell Medal (1972) |
Georgia Totto O'Keeffe (November 15, 1887 – March 6, 1986) was an American modernist artist. She was known for her paintings of enlarged flowers, New York skyscrapers, and New Mexico landscapes. O'Keeffe has been called the "Mother of American modernism".[1][2]
https://en.wikipedia.org/wiki/Georgia_O%27Keeffe
Phantom cats, also known as alien big cats (ABCs), are large felids which allegedly appear in regions outside their indigenous range. Sightings, tracks, and predation have been reported in a number of countries including Australia, Canada, China, Denmark, Finland, France, Germany, Great Britain, Ireland, India, Italy, Luxembourg, Netherlands, New Zealand, Spain, Switzerland, and the United States.
https://en.wikipedia.org/wiki/Phantom_cat#Blue_Mountains_panther
In Australian folklore, the Blue Mountains panther or Lithgow panther is a big cat said to exist by residents of the Blue Mountains area, west of Sydney, New South Wales, for over a century.
Theories suggest the animal may be a descendant of big cats released by World War II US soldiers, which had been used as military mascots.[1] Alternatively, accounts of big cats released by travelling circuses, or which had escaped circuses, exist; as well as rumours that big cats were available for black market purchase in New South Wales in the 20th century.[1]
Over 500 sightings in 20 years have been reported of the panther.[1] In 2018, Grant Denyer and his wife, television producer Cheryl Rogers, reported to have sighted the cat on their property.[2] Photographs have also been recorded in Gippsland and Hawkesbury.[3] In 2002, a Kenthurst teenager Luke Walker was attacked by what he stated to be a large feline; he suffered deep lacerations.[4] Sightings have been made by a NSW Police Force detective, an officer of the Department of Agriculture, and Rural Fire Service personnel, as well as pilots.[4]
Encounters continue to be reported upon in national newspapers as of 2020, when video footage was allegedly recorded in the grounds of Sydney Adventist Hospital, Wahroonga[5][6] Most recently in April 2020, the Go Pro-associated Instagram collective bluemtns_explore shared photographs of alleged big cat tracks discovered during a mountain trek.[7] The Grose Vale Group, which has collected reports of sightings since 1998, claims it collects 20 to 30 sightings a year.[8]
https://en.wikipedia.org/wiki/Blue_Mountains_panther
| Roborovski hamster | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Rodentia |
| Family: | Cricetidae |
| Subfamily: | Cricetinae |
| Genus: | Phodopus |
| Species: | P. roborovskii
|
| Binomial name | |
| Phodopus roborovskii (Satunin, 1903)
| |

| |
| Distribution of P. roborovskii | |
| Synonyms | |
| |
The Roborovski hamster (Phodopus roborovskii), also known as the desert hamster, Robo dwarf hamster, or simply dwarf hamster, is the smallest of three species of hamster in the genus Phodopus. It lives in the deserts of Central Asia, averaging 1.8 centimetres (0.7 in) at birth and 4.8 centimetres (1.9 in) and 12–24 grams (0.42–0.85 oz) during adulthood.[2] Distinguishing characteristics of the Roborovskis are eyebrow-like white spots and the lack of any dorsal stripe (found on the other members of the genus Phodopus). The average lifespan for the Roborovski hamster is 2–3 years, though this is dependent on living conditions (extremes being four years in captivity and two in the wild).[3] Roborovskis are known for their speed and have been said to run up to 6 miles a night.[4]
https://en.wikipedia.org/wiki/Roborovski_dwarf_hamster
Red Mountain is a long ridge running southwest-northeast and dividing Jones Valley from Shades Valley south of Birmingham, Alabama. It is part of the Ridge-and-Valley region of the Appalachian mountains. The Red Mountain Formation of hard Silurian rock strata lies exposed in several long crests, and was named "Red Mountain" because of the rust-stained rock faces and prominent seams of red hematite iron ore. The mountain was the site of the Sloss, Republic Steel, Woodward Iron and Tennessee Coal and iron mines which supplied ore to Birmingham's iron furnaces. The best displays of the mountain's geological strata occur at the Twentieth Street cut near the Vulcan statue and at the U.S. Route 31 highway cut leading into the suburb of Homewood. Most of Birmingham's television and radio stations have their transmission towers located on Red Mountain.
Red Mountain is also home to Red Mountain Park, one of the largest urban parks in the United States at 1,500 acres (6.1 km2).
https://en.wikipedia.org/wiki/Red_Mountain_(Birmingham)
Cavite | |
|---|---|
| Province of Cavite | |
Clockwise (from the top): Aguinaldo Shrine, monolith of Mount Pico de Loro, Tagaytay, Corregidor Island, monument of the Thirteen Martyrs of Cavite | |
| Nickname: Historical Capital of the Philippines[1] | |
| Motto(s): | |
| Anthem: Filipino: Himno ng Kabite English: (Cavite Hymn) | |
 Location in the Philippines | |
OpenStreetMap | |
| Coordinates: 14°16′N 120°52′ECoordinates: 14°16′N 120°52′E | |
| Country | Philippines |
|---|---|
| Region | Calabarzon |
| Established | 1614[2][3] |
| Capital |
|
| Largest city | Dasmariñas |
| Government | |
| • Type | Sangguniang Panlalawigan |
| • Governor | Jonvic Remulla (NUP) |
| • Vice Governor | Athena Bryana Tolentino (NUP) |
| • Legislature | Cavite Provincial Board |
| Area | |
| • Total | 1,574.17 km2 (607.79 sq mi) |
| • Land | 1,426.06 km2 (550.60 sq mi) |
| • Rank | 67th out of 81 |
| Highest elevation | 688 m (2,257 ft) |
| Population (2020 census)[7] | |
| • Total | 4,344,829 |
| • Rank | 1st out of 81 |
| • Density | 2,800/km2 (7,100/sq mi) |
| • Rank | 2nd out of 81 |
| Demonym(s) | Caviteño (masculine or neutral) Caviteña (feminine) |
| Divisions | |
| • Independent cities | 0 |
| • Component cities | |
7 | |
| • Municipalities |
|---|
16 | |
| • Barangays | 829 |
|---|---|
| • Districts | Legislative districts of Cavite |
| Demographics | |
| • Ethnic groups | |
| • Native languages | Tagalog Chavacano |
| • Languages | (Major language) Filipino English Chavacano (Minor language) Bicolano Cebuano Ilocano Hiligaynon Waray |
| • Major religions | |
| • Feast date | 2nd and 3rd Sunday of November |
| • Ecclesiastical dioceses |
|
| • Patron saint | |
| Time zone | UTC+8 (PHT) |
| IDD : area code | +63 (0)46 |
| ISO 3166 code | PH-CAV |
| Website | www |
Cavite, officially the Province of Cavite (Tagalog: Lalawigan ng Kabite;[b] Chavacano: Provincia de Cavite), is a province in the Philippines located in the Calabarzon region in Luzon. Located on the southern shores of Manila Bay and southwest of Manila, it is one of the most industrialized and fastest-growing provinces in the Philippines. As of 2020, it has a population of 4,344,829, making it the most populated province in the country if the independent cities of Cebu are excluded from Cebu's population figure.
The de facto capital and seat of the government of the province is Trece Martires, although Imus is the official (de jure) capital while the City of Dasmariñas is the largest city in the province.
For over 300 years, the province played an important role in both the country's colonial past and eventual fight for independence, earning it the title "Historical Capital of the Philippines". It became the cradle of the Philippine Revolution, which led to the renouncement of Spanish colonial control, finally culminating in the Philippine Declaration of Independence on June 12, 1898 in Kawit. The old provincial capital, Cavite City also hosted docks for the Manila galleon, becoming an essential part of commerce between Asia and Latin America.
Originally an agricultural province, its northern cities of Bacoor, Imus, and Dasmariñas (with a combined population of 1,864,560 at the 2020 Census) are now suburbs of Manila due to increasing urbanization in the late 1900s. This province forms part of the Greater Manila Area.
https://en.wikipedia.org/wiki/Cavite
 | |
| Use | National flag and ensign |
|---|---|
| Proportion | 10:19 |
| Adopted | 1968 |
| Design | Large Rainbow compassing the Flag, four Mountains one White, Blue, Yellow and Black; Navajo Reservation outline in Copper Orange. |
The flag of the Navajo Nation is the official flag of the Navajo Nation, a Native American governed nation in the Four Corners states of Arizona, New Mexico, Colorado, and Utah.[1]
https://en.wikipedia.org/wiki/Flag_of_the_Navajo_Nation
| Llyn y Fan Fach | |
|---|---|
 | |
| Location | Brecon Beacons National Park, Carmarthenshire: View from near Picws Du |
| Coordinates | 51°52′55″N 3°44′31″WCoordinates: 51°52′55″N 3°44′31″W |
| Type | reservoir, natural lake |
| Primary outflows | River Sawdde |
| Basin countries | United Kingdom |
| Max. depth | 29 m (95 ft) |
| Surface elevation | 506 m (1,660 ft) |
Llyn y Fan Fach (Welsh meaning "little lake (near) the peak")[1] is a lake of approximately 10 hectares (25 acres) on the northern margin of the Black Mountain in Carmarthenshire, South Wales and lying within the Brecon Beacons National Park. The lake lies at an altitude of approximately 1,660 feet (510 m), immediately to the north of the ridge of the Carmarthen Fans. It is the smaller of two lakes within this mountain massif: the slightly larger Llyn y Fan Fawr is about 2 miles (3.2 km) to the east.
https://en.wikipedia.org/wiki/Llyn_y_Fan_Fach
Oil sands, tar sands, crude bitumen, or bituminous sands, are a type of unconventional petroleum deposit. Oil sands are either loose sands or partially consolidated sandstone containing a naturally occurring mixture of sand, clay, and water, soaked with bitumen, a dense and extremely viscous form of petroleum.
Significant bitumen deposits are reported in Canada,[1][2] Kazakhstan, Russia, and Venezuela. The estimated worldwide deposits of oil are more than 2 trillion barrels (320 billion cubic metres);[3]. Proven reserves of bitumen contain approximately 100 billion barrels,[4] and total natural bitumen reserves are estimated at 249.67 Gbbl (39.694×109 m3) worldwide, of which 176.8 Gbbl (28.11×109 m3), or 70.8%, are in Alberta, Canada.[1]
Crude bitumen is a thick, sticky form of crude oil, so viscous that it will not flow unless heated or diluted with lighter hydrocarbons such as light crude oil or natural-gas condensate. At room temperature, it is much like cold molasses.[5] The Orinoco Belt in Venezuela is sometimes described as oil sands, but these deposits are non-bituminous, falling instead into the category of heavy or extra-heavy oil due to their lower viscosity.[6] Natural bitumen and extra-heavy oil differ in the degree by which they have been degraded from the original conventional oils by bacteria.
https://en.wikipedia.org/wiki/Oil_sands
https://en.wikipedia.org/wiki/Grand_Staircase%E2%80%93Escalante_National_Monument
https://en.wikipedia.org/wiki/Northern_rough-winged_swallow
https://en.wikipedia.org/wiki/Limestone
https://en.wikipedia.org/wiki/Beetle
https://en.wikipedia.org/wiki/Volcano
https://en.wikipedia.org/wiki/Escudilla_Mountain
| African wildcat | |
|---|---|

| |
| An African wildcat at Parc des Félins | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Carnivora |
| Suborder: | Feliformia |
| Family: | Felidae |
| Subfamily: | Felinae |
| Genus: | Felis |
| Species: | F. lybica
|
| Binomial name | |
| Felis lybica Forster, 1780
| |
| Subspecies | |
| |

| |
| Distribution of the African wildcat as of 2015[1] | |
The African wildcat (Felis lybica) is a small wildcat species with sandy grey fur, pale vertical stripes on the sides and around the face. It is native to Africa, West and Central Asia, and is distributed to Rajasthan in India and Xinjiang in China. It inhabits a broad variety of landscapes ranging from deserts to savannas, shrublands and grasslands.
https://en.wikipedia.org/wiki/African_wildcat






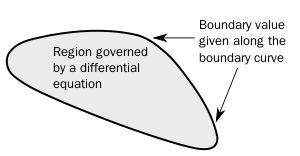

























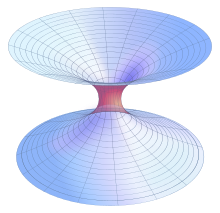




































































































































































No comments:
Post a Comment