Animal breeding is a branch of animal science that addresses the evaluation (using best linear unbiased prediction and other methods) of the genetic value (estimated breeding value, EBV) of livestock. Selecting for breeding animals with superior EBV in growth rate, egg, meat, milk, or wool production, or with other desirable traits has revolutionized livestock production throughout the entire world. The scientific theory of animal breeding incorporates population genetics, quantitative genetics, statistics, and recently molecular genetics and is based on the pioneering work of Sewall Wright, Jay Lush, and Charles Henderson.
Breeding stock
Breeding stock is a group of animals used for the purpose of planned breeding. When individuals are looking to breed animals, they look for certain valuable traits in purebred animals, or may intend to use some type of crossbreeding to produce a new type of stock with different, and presumably super abilities in a given area of endeavor. For example, when breeding swine for meat, the "breeding stock should be sound, fast growing, muscular, lean, and reproductively efficient."[1] The "subjective selection of breeding stock" in horses has led to many horse breeds with particular performance traits.[2] While breeding animals is common in an agricultural setting, it is also a common practice for the purpose of selling animals meant as pets, such as cats, dogs, horses, and birds, as well as less common animals, such as reptiles or some primates.
Purebred breeding
Mating animals of the same breed for maintaining such breed is referred to as purebred breeding. Opposite to the practice of mating animals of different breeds, purebred breeding aims to establish and maintain stable traits, that animals will pass to the next generation. By "breeding the best to the best", employing a certain degree of inbreeding, considerable culling, and selection for "superior" qualities, one could develop a bloodline or "breed" superior in certain respects to the original base stock.
Such animals can be recorded with a breed registry, the organisation that maintains pedigrees and/or stud books. The observable phenomenon of hybrid vigor stands in contrast to the notion of breed purity.
For laboratory purposes, organisms such as mice have been inbred to 100% pure lines, as offered for sale by the Jackson laboratory. But this is highly unusual and difficult to do for most organisms, in whose populations all individuals harbor recessive, deleterious gene variants (alleles).
Backyard breeding
In the United States, a backyard breeder is someone who breeds animals, often without registration and with a focus on profit. In some cases, the animals are inbred narrowly for looks, with little regard to health.[3] The term is considered derogatory. If a backyard dog breeder has a significant number of breeding animals, they become associated with puppy mills. Most puppy mills are licensed with the USDA.[4]
See also
Plant and animal breeding
- Artificial insemination of livestock and pets
- Artificial selection
- Agricultural science
- Backyard breeder
- Genomics of domestication
- Plant breeding
- Progeny testing
- Selective breeding
People
Other topics
- BLUPF90 and ASReml: software packages for animal breeding statistics
- Veterinary medicine
Further reading
- Lush, JL (1937), Animal Breeding Plans, Ames, Iowa: Iowa State Press
- Kempthorne, O (1957), Introduction to Statistic Genetics, John Wiley & Sons
- Van Vleck; L. D.; Searle; S. R. (1979), Variance components and animal breeding: proceedings of a conference in honor of C.R. Henderson, Ithaca, N.Y.: Cornell University
- Henderson, CR (1984), Applications of linear models in animal breeding, Guelph, Ont: University of Guelph, ISBN 0-88955-030-1
- Hammond K. Gianola, D. (1990), Advances in Statistical Methods for Genetic Improvement of Livestock (Advanced Series in Agricultural Sciences), Springer-Verlag Berlin and Heidelberg GmbH & Co. K, ISBN 3-540-50809-0
- Massey, JW; Vogt, DW (1993), Heritability and Its Use in Animal Breeding, Department of Animal Sciences, University of Missouri
- Mrode, R. A. (1996), Linear models for the prediction of animal breeding values, Oxon: CAB International, ISBN 0-85198-996-9
- Cameron, N. D. (1997), Selection indices and prediction of genetic merit in animal breeding, Oxon: CAB International, ISBN 0-85199-169-6
- Dalton, C; Willis, MB (1998), Dalton's Introduction to Practical Animal Breeding, Oxford: Blackwell Science, ISBN 0-632-04947-2
- Bourdon, RM (2000), Understanding animal breeding, Englewood Cliffs, N.J: Prentice Hall, ISBN 0-13-096449-2
- Newman, S; Rothschild, MF (2002), Intellectual Property Rights in Animal Breeding and Genetics, Wallingford, Oxon, UK: CABI Pub, ISBN 0-85199-641-8
- FAO. (2007). The Global Plan of Action for Animal Genetic Resources and the Interlaken Declaration. Rome.
- FAO. (2010). Breeding strategies for sustainable management of animal genetic resources. FAO Animal Production and Health Guidelines. No. 3. Rome.
- FAO. (2015). The Second Report on the State of the World's Animal Genetic Resources for Food and Agriculture. Rome.
References
External links
- Domestic Animal Diversity Information System
- Guidelines For Uniform Swine Improvement Programs, National Swine Improvement Federation, 2003, archived from the original on 2008-10-21
- Implementing the Global Plan of Action for Animal Genetic Resources
- bataille de la somme
Academic centers
- Animal Breeding and Genomics Centre, Wageningen University, Netherlands, 23 March 2022
- Animal Breeding and Genetics Group, University of Georgia, USA
- Centre for Genetic Improvement of Livestock, University of Guelph, Canada
- Animal Breeding & Genetics, Cornell University, USA
- Animal Breeding & Genetics, Iowa State University, USA
- Breeding and Genetics Program, Colorado State University, USA, archived from the original on 2008-10-12
- Animal Genetics and Breeding Unit, University of New England, Australia
- European Graduate School in Animal Breeding and Genetics (EGS-ABG), AgroParisTech, France ; Wageningen University, Netherlands ; Aarhus University, Denmark ; Swedish University of Agricultural Sciences, Sweden
Journals
- "Journal of Animal Breeding and Genetics", Journal of Animal Breeding and Genetics (1986), ISSN 0931-2668, archived from the original on 2008-12-12, retrieved 2008-09-06
Organizations
- Association for the Advancement of Animal Breeding and Genetics, Australia
- Roslin Institute, Scotland
- Animal Breeding, Genetics & Genomics, Cooperative State Research, Education, and Extension Service, USA, archived from the original on 2008-09-20
- Animal Improvement Programs Laboratory, Beltsville Agricultural Research Center, USA, archived from the original on 2008-10-12, retrieved 2008-09-06
- Animal Genetics and Breeding Unit, Australia, archived from the original on 2008-07-19
- INRA, Institut National de la Recherché Agronomique, France
- INIA, Instituto Nacional Investigación Agraria, Spain, archived from the original on 2013-08-12
https://en.wikipedia.org/wiki/Animal_breeding
https://en.wikipedia.org/wiki/Cryopreservation
https://en.wikipedia.org/wiki/Genetic_engineering
Euthanasia (from Greek: εὐθανασία, lit. 'good death': εὖ, eu, 'well, good' + θάνατος, thanatos, 'death') is the practice of intentionally ending life to eliminate pain and suffering.[1][2]
https://en.wikipedia.org/wiki/Euthanasia
Cremation is a method of final disposition of a dead body through burning.[1]
https://en.wikipedia.org/wiki/Cremation
Decapitation or beheading is the total separation of the head from the body. Such an injury is invariably fatal to humans and most other animals, since it deprives the brain of oxygenated blood, while all other organs are deprived of the involuntary functions that are needed for the body to function.
https://en.wikipedia.org/wiki/Decapitation
https://en.wikipedia.org/wiki/sword
This article needs additional citations for verification. (August 2018) |
Plant breeding is the science of changing the traits of plants in order to produce desired characteristics.[1] It has been used to improve the quality of nutrition in products for humans and animals.[2] The goals of plant breeding are to produce crop varieties that boast unique and superior traits for a variety of applications. The most frequently addressed agricultural traits are those related to biotic and abiotic stress tolerance, grain or biomass yield, end-use quality characteristics such as taste or the concentrations of specific biological molecules (proteins, sugars, lipids, vitamins, fibers) and ease of processing (harvesting, milling, baking, malting, blending, etc.).[3]
Plant breeding can be performed through many different techniques ranging from simply selecting plants with desirable characteristics for propagation, to methods that make use of knowledge of genetics and chromosomes, to more complex molecular techniques. Genes in a plant are what determine what type of qualitative or quantitative traits it will have. Plant breeders strive to create a specific outcome of plants and potentially new plant varieties,[2] and in the course of doing so, narrow down the genetic diversity of that variety to a specific few biotypes.[4]
It is practiced worldwide by individuals such as gardeners and farmers, and by professional plant breeders employed by organizations such as government institutions, universities, crop-specific industry associations or research centers. International development agencies believe that breeding new crops is important for ensuring food security by developing new varieties that are higher yielding, disease resistant, drought tolerant or regionally adapted to different environments and growing conditions.[5]
A recent study shows that without plant breeding, Europe would have produced 20% fewer arable crops over the last 20 years, consuming an additional 21.6 million hectares of land and emitting 4 billion tonnes of carbon.[6][7] Wheat species created for Morocco are currently being crossed with plants to create new varieties for northern France. Soy beans, which were previously grown predominantly in the south of France, are now grown in southern Germany.[6][8]
History
Plant breeding started with sedentary agriculture and particularly the domestication of the first agricultural plants, a practice which is estimated to date back 9,000 to 11,000 years.[9] Initially early farmers simply selected food plants with particular desirable characteristics, and employed these as progenitors for subsequent generations, resulting in an accumulation of valuable traits over time.
Grafting technology had been practiced in China before 2000 BCE.[10]
By 500 BCE grafting was well established and practiced.[11]
Gregor Mendel (1822–84) is considered the "father of genetics". His experiments with plant hybridization led to his establishing laws of inheritance. Genetics stimulated research to improve crop production through plant breeding.
Modern plant breeding is applied genetics, but its scientific basis is broader, covering molecular biology, cytology, systematics, physiology, pathology, entomology, chemistry, and statistics (biometrics). It has also developed its own technology.
Classical plant breeding
This section needs additional citations for verification. (December 2011) |
One major technique of plant breeding is selection, the process of selectively propagating plants with desirable characteristics and eliminating or "culling" those with less desirable characteristics.[12]
Another technique is the deliberate interbreeding (crossing) of closely or distantly related individuals to produce new crop varieties or lines with desirable properties. Plants are crossbred to introduce traits/genes from one variety or line into a new genetic background. For example, a mildew-resistant pea may be crossed with a high-yielding but susceptible pea, the goal of the cross being to introduce mildew resistance without losing the high-yield characteristics. Progeny from the cross would then be crossed with the high-yielding parent to ensure that the progeny were most like the high-yielding parent, (backcrossing). The progeny from that cross would then be tested for yield (selection, as described above) and mildew resistance and high-yielding resistant plants would be further developed. Plants may also be crossed with themselves to produce inbred varieties for breeding. Pollinators may be excluded through the use of pollination bags.
Classical breeding relies largely on homologous recombination between chromosomes to generate genetic diversity. The classical plant breeder may also make use of a number of in vitro techniques such as protoplast fusion, embryo rescue or mutagenesis (see below) to generate diversity and produce hybrid plants that would not exist in nature.
Traits that breeders have tried to incorporate into crop plants include:
- Improved quality, such as increased nutrition, improved flavor, or greater beauty
- Increased yield of the crop
- Increased tolerance of environmental pressures (salinity, extreme temperature, drought)
- Resistance to viruses, fungi and bacteria
- Increased tolerance to insect pests
- Increased tolerance of herbicides
- Longer storage period for the harvested crop
Before World War II
Successful commercial plant breeding concerns were founded from the late 19th century.[clarification needed] Gartons Agricultural Plant Breeders in England was established in the 1890s by John Garton, who was one of the first to commercialize new varieties of agricultural crops created through cross-pollination.[13] The firm's first introduction was the Abundance Oat, an oat variety.[14][15] It is one of the first agricultural grain varieties bred from a controlled cross, introduced to commerce in 1892.[14][15]
In the early 20th century, plant breeders realized that Gregor Mendel's findings on the non-random nature of inheritance could be applied to seedling populations produced through deliberate pollinations to predict the frequencies of different types. Wheat hybrids were bred to increase the crop production of Italy during the so-called "Battle for Grain" (1925–1940). Heterosis was explained by George Harrison Shull. It describes the tendency of the progeny of a specific cross to outperform both parents. The detection of the usefulness of heterosis for plant breeding has led to the development of inbred lines that reveal a heterotic yield advantage when they are crossed. Maize was the first species where heterosis was widely used to produce hybrids.
Statistical methods were also developed to analyze gene action and distinguish heritable variation from variation caused by environment. In 1933 another important breeding technique, cytoplasmic male sterility (CMS), developed in maize, was described by Marcus Morton Rhoades. CMS is a maternally inherited trait that makes the plant produce sterile pollen. This enables the production of hybrids without the need for labor-intensive detasseling.
These early breeding techniques resulted in large yield increase in the United States in the early 20th century. Similar yield increases were not produced elsewhere until after World War II, the Green Revolution increased crop production in the developing world in the 1960s.
After World War II
Following World War II a number of techniques were developed that allowed plant breeders to hybridize distantly related species, and artificially induce genetic diversity.
When distantly related species are crossed, plant breeders make use of a number of plant tissue culture techniques to produce progeny from otherwise fruitless mating. Interspecific and intergeneric hybrids are produced from a cross of related species or genera that do not normally sexually reproduce with each other. These crosses are referred to as Wide crosses. For example, the cereal triticale is a wheat and rye hybrid. The cells in the plants derived from the first generation created from the cross contained an uneven number of chromosomes and as a result was sterile. The cell division inhibitor colchicine was used to double the number of chromosomes in the cell and thus allow the production of a fertile line.
Failure to produce a hybrid may be due to pre- or post-fertilization incompatibility. If fertilization is possible between two species or genera, the hybrid embryo may abort before maturation. If this does occur the embryo resulting from an interspecific or intergeneric cross can sometimes be rescued and cultured to produce a whole plant. Such a method is referred to as embryo rescue. This technique has been used to produce new rice for Africa, an interspecific cross of Asian rice Oryza sativa and African rice Oryza glaberrima.
Hybrids may also be produced by a technique called protoplast fusion. In this case protoplasts are fused, usually in an electric field. Viable recombinants can be regenerated in culture.
Chemical mutagens like ethyl methanesulfonate (EMS) and dimethyl sulfate (DMS), radiation, and transposons are used for mutagenesis. Mutagenesis is the generation of mutants. The breeder hopes for desirable traits to be bred with other cultivars – a process known as Mutation Breeding. Classical plant breeders also generate genetic diversity within a species by exploiting a process called somaclonal variation, which occurs in plants produced from tissue culture, particularly plants derived from callus. Induced polyploidy, and the addition or removal of chromosomes using a technique called chromosome engineering may also be used.
When a desirable trait has been bred into a species, a number of crosses to the favored parent are made to make the new plant as similar to the favored parent as possible. Returning to the example of the mildew resistant pea being crossed with a high-yielding but susceptible pea, to make the mildew resistant progeny of the cross most like the high-yielding parent, the progeny will be crossed back to that parent for several generations (See backcrossing). This process removes most of the genetic contribution of the mildew resistant parent. Classical breeding is therefore a cyclical process.[clarification needed]
With classical breeding techniques, the breeder does not know exactly what genes have been introduced to the new cultivars. Some scientists therefore argue that plants produced by classical breeding methods should undergo the same safety testing regime as genetically modified plants. There have been instances where plants bred using classical techniques have been unsuitable for human consumption, for example the poison solanine was unintentionally increased to unacceptable levels in certain varieties of potato through plant breeding. New potato varieties are often screened for solanine levels before reaching the marketplace.[citation needed]
Even with the very latest in biotech-assisted conventional breeding, incorporation of a trait takes an average of seven generations for clonally propagated crops, nine for self-fertilising, and seventeen for cross-pollinating.[16][17]
Modern plant breeding
Modern plant breeding may use techniques of molecular biology to select, or in the case of genetic modification, to insert, desirable traits into plants. Application of biotechnology or molecular biology is also known as molecular breeding.
Marker assisted selection
Sometimes many different genes can influence a desirable trait in plant breeding. The use of tools such as molecular markers or DNA fingerprinting can map thousands of genes. This allows plant breeders to screen large populations of plants for those that possess the trait of interest. The screening is based on the presence or absence of a certain gene as determined by laboratory procedures, rather than on the visual identification of the expressed trait in the plant. The purpose of marker assisted selection, or plant genome analysis, is to identify the location and function (phenotype) of various genes within the genome. If all of the genes are identified it leads to genome sequence.[citation needed][clarification needed] All plants have varying sizes and lengths of genomes with genes that code for different proteins, but many are also the same. If a gene's location and function is identified in one plant species, a very similar gene likely can also be found in a similar location in another related species genome.[18]
Reverse breeding and doubled haploids (DH)
This section may be confusing or unclear to readers. In particular, some explanation of reverse breeding is still missing here. (March 2017) |
Homozygous plants with desirable traits can be produced from heterozygous starting plants, if a haploid cell with the alleles for those traits can be produced, and then used to make a doubled haploid. The doubled haploid will be homozygous for the desired traits. Furthermore, two different homozygous plants created in that way can be used to produce a generation of F1 hybrid plants which have the advantages of heterozygosity and a greater range of possible traits. Thus, an individual heterozygous plant chosen for its desirable characteristics can be converted into a heterozygous variety (F1 hybrid) without the necessity of vegetative reproduction but as the result of the cross of two homozygous/doubled haploid lines derived from the originally selected plant.[19] Plant tissue culturing can produce haploid or double haploid plant lines and generations. This cuts down the genetic diversity taken from that plant species in order to select for desirable traits that will increase the fitness of the individuals. Using this method decreases the need for breeding multiple generations of plants to get a generation that is homogeneous for the desired traits, thereby saving much time over the natural version of the same process. There are many plant tissue culturing techniques that can be used to achieve haploid plants, but microspore culturing is currently the most promising for producing the largest numbers of them.[18]
Genetic modification
Genetic modification of plants is achieved by adding a specific gene or genes to a plant, or by knocking down a gene with RNAi, to produce a desirable phenotype. The plants resulting from adding a gene are often referred to as transgenic plants. If for genetic modification genes of the species or of a crossable plant are used under control of their native promoter, then they are called cisgenic plants. Sometimes genetic modification can produce a plant with the desired trait or traits faster than classical breeding because the majority of the plant's genome is not altered.
To genetically modify a plant, a genetic construct must be designed so that the gene to be added or removed will be expressed by the plant. To do this, a promoter to drive transcription and a termination sequence to stop transcription of the new gene, and the gene or genes of interest must be introduced to the plant. A marker for the selection of transformed plants is also included. In the laboratory, antibiotic resistance is a commonly used marker: Plants that have been successfully transformed will grow on media containing antibiotics; plants that have not been transformed will die. In some instances markers for selection are removed by backcrossing with the parent plant prior to commercial release.
The construct can be inserted in the plant genome by genetic recombination using the bacteria Agrobacterium tumefaciens or A. rhizogenes, or by direct methods like the gene gun or microinjection. Using plant viruses to insert genetic constructs into plants is also a possibility, but the technique is limited by the host range of the virus. For example, Cauliflower mosaic virus (CaMV) only infects cauliflower and related species. Another limitation of viral vectors is that the virus is not usually passed on to the progeny, so every plant has to be inoculated.
The majority of commercially released transgenic plants are currently limited to plants that have introduced resistance to insect pests and herbicides. Insect resistance is achieved through incorporation of a gene from Bacillus thuringiensis (Bt) that encodes a protein that is toxic to some insects. For example, the cotton bollworm, a common cotton pest, feeds on Bt cotton it will ingest the toxin and die. Herbicides usually work by binding to certain plant enzymes and inhibiting their action.[20] The enzymes that the herbicide inhibits are known as the herbicides target site. Herbicide resistance can be engineered into crops by expressing a version of target site protein that is not inhibited by the herbicide. This is the method used to produce glyphosate resistant ("Roundup Ready") crop plants.
Genetic modification can further increase yields by increasing stress tolerance to a given environment. Stresses such as temperature variation, are signalled to the plant via a cascade of signalling molecules which will activate a transcription factor to regulate gene expression. Overexpression of particular genes involved in cold acclimation has been shown to produce more resistance to freezing, which is one common cause of yield loss[21]
Genetic modification of plants that can produce pharmaceuticals (and industrial chemicals), sometimes called pharming, is a rather radical new area of plant breeding.[22]
The debate surrounding genetically modified food during the 1990s peaked in 1999 in terms of media coverage and risk perception,[23] and continues today – for example, "Germany has thrown its weight behind a growing European mutiny over genetically modified crops by banning the planting of a widely grown pest-resistant corn variety."[24] The debate encompasses the ecological impact of genetically modified plants, the safety of genetically modified food and concepts used for safety evaluation like substantial equivalence. Such concerns are not new to plant breeding. Most countries have regulatory processes in place to help ensure that new crop varieties entering the marketplace are both safe and meet farmers' needs. Examples include variety registration, seed schemes, regulatory authorizations for GM plants, etc.
Breeding and the microbiome
Industrial breeding of plants has unintentionally altered how agricultural cultivars associate with their microbiome.[25] In maize, for example, breeding has altered the nitrogen cycling taxa required to the rhizosphere, with more modern lines recruiting less nitrogen fixing taxa and more nitrifiers and denitrifiers.[26] Microbiomes of breeding lines showed that hybrid plants share much of their bacterial community with their parents, such as Cucurbita seeds and apple shoot endophytes.[27][28][29] In addition, the proportional contribution of the microbiome from parents to offspring corresponds to the amount of genetic material contributed by each parent during breeding and domestication.[29]
Phenotyping and artificial intelligence
As of 2020 machine learning – and especially deep machine learning – has recently become more commonly used in phenotyping. Computer vision using ML has made great strides and is now being applied to leaf phenotyping and other phenotyping jobs typically performed by human eyes. Pound et al. 2017 and Singh et al. 2016 are especially salient examples of early successful application and demonstration of the general usability of the process across multiple target plant species. These methods will work even better with large, publicly available open data sets.[30]
Speed breeding
Speed breeding is introduced by Watson et al. 2018. Classical (human performed) phenotyping during speed breeding is also possible, using a procedure developed by Richard et al. 2015. As of 2020 it is highly anticipated that SB and automated phenotyping will, combined, produce greatly improved outcomes – see § Phenotyping and artificial intelligence above.[30]
Genomic selection (GS)
The NGS platform has substantially declined the time and cost required for sequencing and facilitated SNP discovery in model and non-model plants. This in turn has led to employing large-scale SNP markers in genomic selection approaches which aim at predicting genomic breeding values/GEBVs of genotypes in a given population. This method can increase the selection accuracy and decrease the time of each breeding cycle. It has been used in different crops such as maize, wheat, etc.[31][32]
Participatory plant breeding
Participatory plant breeding (PPB) is when farmers are involved in a crop improvement programme with opportunities to make decisions and contribute to the research process at different stages.[33][34][35] Participatory approaches to crop improvement can also be applied when plant biotechnologies are being used for crop improvement.[36] Local agricultural systems and genetic diversity are strengthened by participatory programs, and outcomes are enhanced by farmers knowledge of the quality required and evaluation of the target environment.[37]
A 2019 review of participatory plant breeding indicated that it had not gained widespread acceptance despite its record of successfully developing varieties with improved diversity and nutritional quality, as well as greater likelihood of these improved varieties being adopted by farmers. This review also found participatory plant breeding to have a better cost/benefit ratio than non-participatory approaches, and suggested incorporating participatory plant breeding with evolutionary plant breeding.[38]
Evolutionary plant breeding
Evolutionary plant breeding describes practices which use mass populations with diverse genotypes grown under competitive natural selection. Survival in common crop cultivation environments is the predominant method of selection, rather than direct selection by growers and breeders. Individual plants that are favored under prevailing growing conditions, such as environment and inputs, contribute more seed to the next generation than less-adapted individuals.[39] Evolutionary plant breeding has been successfully used by the Nepal National Gene Bank to preserve landrace diversity within Jumli Marshi rice while reducing its susceptibility to blast disease. These practices have also been used in Nepal with bean landraces.[40]
In 1929, Harlan and Martini proposed a method of plant breeding with heterogeneous populations by pooling an equal number of F2 seeds obtained from 378 crosses among 28 geographically diverse barley cultivars. In 1938, Harlan and Martini demonstrated evolution by natural selection in mixed dynamic populations as a few varieties that became dominant in some locations almost disappeared in others; poorly-adapted varieties disappeared everywhere.[41]
Evolutionary breeding populations have been used to establish self-regulating plant–pathogen systems. Examples include barley, where breeders were able to improve resistance to Rynchosporium secalis scald over 45 generations.[42] An evolutionary breeding project grew F5 hybrid bulk soybean populations on soil infested by the soybean cyst nematode and was able to increase the proportion of resistant plants from 5% to 40%. The International Center for Agricultural Research in the Dry Areas (ICARDA) evolutionary plant breeding is combined with participatory plant breeding in order to allow farmers to choose which varieties suit their needs in their local environment.[42]
An influential 1956 effort by Coit A. Suneson to codify this approach coined the term evolutionary plant breeding and concluded that 15 generations of natural selection are desirable to produce results that are competitive with conventional breeding.[43] Evolutionary breeding allows working with much larger plant population sizes than conventional breeding.[41] It has also been used in tandem with conventional practices in order to develop both heterogeneous and homogeneous crop lines for low input agricultural systems that have unpredictable stress conditions.[44]
Evolutionary plant breeding has been delineated into four stages:[39]
- Stage 1: Genetic diversity is created, for example by manual crosses of inbreeding species or mixing of cultivars in outcrossing species.
- Stage 2: Multiplication of seeds
- Stage 3: Seeds of each cross are then mixed to produce the first generation of the Composite Cross Population (CCP). The entire offspring is sown to grow and set seed. As the number of plants in the population increases, a proportion of the harvested seed is saved for sowing.
- Stage 4: The seed can be used as a for continued evolutionary plant breeding or as a starting point for a conventional breeding effort.
Issues and concerns
Breeding and food security
Issues facing plant breeding in the future include the lack of arable land, increasingly harsh cropping conditions and the need to maintain food security, which involves being able to provide the world population with sufficient nutrition. Crops need to be able to mature in multiple environments to allow worldwide access, which involves solving problems including drought tolerance. It has been suggested that global solutions are achievable through the process of plant breeding, with its ability to select specific genes allowing crops to perform at a level which yields the desired results.[45] One issue facing agriculture is the loss of landraces and other local varieties which have diversity that may have useful genes for climate adaptation in the future.[42]
Conventional breeding intentionally limits phenotype plasticity within genotypes and limits variability between genotypes.[44] Uniformity does not allow crops to adapt to climate change and other biotic stresses and abiotic stresses.[42]
Plant breeders rights
Plant breeders' rights is an important and controversial issue. Production of new varieties is dominated by commercial plant breeders, who seek to protect their work and collect royalties through national and international agreements based in intellectual property rights. The range of related issues is complex. In the simplest terms, critics of the increasingly restrictive regulations argue that, through a combination of technical and economic pressures, commercial breeders are reducing biodiversity and significantly constraining individuals (such as farmers) from developing and trading seed on a regional level.[46] Efforts to strengthen breeders' rights, for example, by lengthening periods of variety protection, are ongoing.[citation needed]
Intellectual property legislation for plants often uses definitions that typically include genetic uniformity and unchanging appearance over generations. These legal definitions of stability contrast with traditional agronomic usage, which considers stability in terms of how consistent the yield or quality of a crop remains across locations and over time.[39]
As of 2020, regulations in Nepal only allow uniform varieties to be registered or released. Evolutionary plant populations and many landraces are polymorphic and do not meet these standards.[40]
Environmental stressors
Uniform and genetically stable cultivars can be inadequate for dealing with environmental fluctuations and novel stress factors.[39] Plant breeders have focused on identifying crops which will ensure crops perform under these conditions; a way to achieve this is finding strains of the crop that is resistance to drought conditions with low nitrogen. It is evident from this that plant breeding is vital for future agriculture to survive as it enables farmers to produce stress resistant crops hence improving food security.[47] In countries that experience harsh winters such as Iceland, Germany and further east in Europe, plant breeders are involved in breeding for tolerance to frost, continuous snow-cover, frost-drought (desiccation from wind and solar radiation under frost) and high moisture levels in soil in winter.[48]
Long-term process
Breeding is not a quick process, which is especially important when breeding to ameliorate a disease. The average time from human recognition of a new fungal disease threat to the release of a resistant crop for that pathogen is at least twelve years.[17][49]
Maintaining specific conditions
When new plant breeds or cultivars are bred, they must be maintained and propagated. Some plants are propagated by asexual means while others are propagated by seeds. Seed propagated cultivars require specific control over seed source and production procedures to maintain the integrity of the plant breeds results. Isolation is necessary to prevent cross contamination with related plants or the mixing of seeds after harvesting. Isolation is normally accomplished by planting distance but in certain crops, plants are enclosed in greenhouses or cages (most commonly used when producing F1 hybrids).
Nutritional value
Modern plant breeding, whether classical or through genetic engineering, comes with issues of concern, particularly with regard to food crops. The question of whether breeding can have a negative effect on nutritional value is central in this respect. Although relatively little direct research in this area has been done, there are scientific indications that, by favoring certain aspects of a plant's development, other aspects may be retarded. A study published in the Journal of the American College of Nutrition in 2004, entitled Changes in USDA Food Composition Data for 43 Garden Crops, 1950 to 1999, compared nutritional analysis of vegetables done in 1950 and in 1999, and found substantial decreases in six of 13 nutrients measured, including 6% of protein and 38% of riboflavin. Reductions in calcium, phosphorus, iron and ascorbic acid were also found. The study, conducted at the Biochemical Institute, University of Texas at Austin, concluded in summary: "We suggest that any real declines are generally most easily explained by changes in cultivated varieties between 1950 and 1999, in which there may be trade-offs between yield and nutrient content."[50]
Plant breeding can contribute to global food security as it is a cost-effective tool for increasing nutritional value of forage and crops. Improvements in nutritional value for forage crops from the use of analytical chemistry and rumen fermentation technology have been recorded since 1960; this science and technology gave breeders the ability to screen thousands of samples within a small amount of time, meaning breeders could identify a high performing hybrid quicker. The genetic improvement was mainly in vitro dry matter digestibility (IVDMD) resulting in 0.7-2.5% increase, at just 1% increase in IVDMD a single Bos Taurus also known as beef cattle reported 3.2% increase in daily gains. This improvement indicates plant breeding is an essential tool in gearing future agriculture to perform at a more advanced level. [51]
Yield
With an increasing population, the production of food needs to increase with it. It is estimated that a 70% increase in food production is needed by 2050 in order to meet the Declaration of the World Summit on Food Security. But with the degradation of agricultural land, simply planting more crops is no longer a viable option. New varieties of plants can in some cases be developed through plant breeding that generate an increase of yield without relying on an increase in land area. An example of this can be seen in Asia, where food production per capita has increased twofold. This has been achieved through not only the use of fertilisers, but through the use of better crops that have been specifically designed for the area.[52][53]
Role of plant breeding in organic agriculture
Some critics of organic agriculture claim it is too low-yielding to be a viable alternative to conventional agriculture in situations when that poor performance may be the result in part of growing poorly-adapted varieties.[54][55] It is estimated that over 95% of organic agriculture is based on conventionally adapted varieties, even though the production environments found in organic vs. conventional farming systems are vastly different due to their distinctive management practices.[55] Most notably, organic farmers have fewer inputs available than conventional growers to control their production environments. Breeding varieties specifically adapted to the unique conditions of organic agriculture is critical for this sector to realize its full potential. This requires selection for traits such as:[55]
- Water use efficiency
- Nutrient use efficiency (particularly nitrogen and phosphorus)
- Weed competitiveness
- Tolerance of mechanical weed control
- Pest/disease resistance
- Early maturity (as a mechanism for avoidance of particular stresses)
- Abiotic stress tolerance (i.e. drought, salinity, etc...)
Currently, few breeding programs are directed at organic agriculture and until recently those that did address this sector have generally relied on indirect selection (i.e. selection in conventional environments for traits considered important for organic agriculture). However, because the difference between organic and conventional environments is large, a given genotype may perform very differently in each environment due to an interaction between genes and the environment (see gene–environment interaction). If this interaction is severe enough, an important trait required for the organic environment may not be revealed in the conventional environment, which can result in the selection of poorly adapted individuals.[54] To ensure the most adapted varieties are identified, advocates of organic breeding now promote the use of direct selection (i.e. selection in the target environment) for many agronomic traits.
There are many classical and modern breeding techniques that can be utilized for crop improvement in organic agriculture despite the ban on genetically modified organisms. For instance, controlled crosses between individuals allow desirable genetic variation to be recombined and transferred to seed progeny via natural processes. Marker assisted selection can also be employed as a diagnostics tool to facilitate selection of progeny who possess the desired trait(s), greatly speeding up the breeding process.[56] This technique has proven particularly useful for the introgression of resistance genes into new backgrounds, as well as the efficient selection of many resistance genes pyramided into a single individual. Unfortunately, molecular markers are not currently available for many important traits, especially complex ones controlled by many genes.
List of notable plant breeders
- Thomas Andrew Knight
- Keith Downey
- Luther Burbank
- Nazareno Strampelli
- Niels Ebbesen Hansen
- Norman Borlaug
- Yvonne Aitken
See also
- Bioactive compound
- Cisgenesis
- Crop
- Crop breeding in Nepal
- Cultivated plant taxonomy
- Double-pair mating
- EUCARPIA
- Family based QTL mapping
- Food security
- Genomics of domestication
- International Code of Nomenclature for Cultivated Plants
- Marker-assisted selection (MAS)
- Orthodox seed
- QTL mapping
- Recalcitrant seed
- Selection methods in plant breeding based on mode of reproduction
- Smart breeding
- Composite cross population
- Bioprospecting / biopiracy / Access and Benefit Sharing Agreement
- Plant Treaty / Convention on Biological Diversity and Nagoya Protocol
- UPOV Convention on New Varieties of Plants
- Farmers rights / Peasants' rights
- Genetic resources (disambiguation)
References
- Lammerts van Bueren, E. T.; G. Backes; H. de Vriend; H. Ostergard (2010). "The role of molecular markers and marker assisted selection in breeding for organic agriculture". Euphytica. 175: 51–64. doi:10.1007/s10681-010-0169-0.
General
- McCouch, S. (2004). "Diversifying Selection in Plant Breeding". PLOS Biol. 2 (10): e347. doi:10.1371/journal.pbio.0020347. PMC 521731. PMID 15486582.
- Briggs, F.N. and Knowles, P.F. 1967. Introduction to Plant Breeding. Reinhold Publishing Corporation, New York.
- Curry, Helen Anne. Evolution Made to Order: Plant Breeding and Technological Innovation in Twentieth-Century America (ISBN 9780226390116) U of Chicago Press, 2016. x, 285 pp.
- Gepts, P. (2002). "A Comparison between Crop Domestication, Classical Plant Breeding, and Genetic Engineering". Crop Science. 42 (6): 1780–1790. doi:10.2135/cropsci2002.1780.
- The Origins of Agriculture and Crop Domestication – The Harlan Symposium
- Schlegel, Rolf (2009) Encyclopedic Dictionary of Plant Breeding 2nd ed. (ISBN 9781439802427), CRC Press, Boca Raton, FL, USA, pp 584
- Schlegel, Rolf (2007) Concise Encyclopedia of Crop Improvement: Institutions, Persons, Theories, Methods, and Histories (ISBN 9781560221463), CRC Press, Boca Raton, FL, USA, pp 423
- Schlegel, Rolf (2014) Dictionary of Plant Breeding, 2nd ed., (ISBN 978-1439802427), CRC Press, Boca Raton, Taylor & Francis Group, Inc., New York, USA, pp 584
- Schouten, Henk J.; Krens, Frans A.; Jacobsen, Evert (2006). "Do cisgenic plants warrant less stringent oversight?". Nature Biotechnology. 24 (7): 753. doi:10.1038/nbt0706-753. PMID 16841052. S2CID 8087798.
- Schouten, Henk J.; Krens, Frans A.; Jacobsen, Evert (2006). "Cisgenic plants are similar to traditionally bred plants". EMBO Reports. 7 (8): 750–753. doi:10.1038/sj.embor.7400769. PMC 1525145. PMID 16880817.
- Sun. "From indica and japonica splitting in common wild rice DNA to the origin and evolution of Asian cultivated rice". Agricultural Archaeology. 1998: 21–29.
- Thro, A.M.; Spillane, C. (1999) Biotechnology assisted participatory plant breeding: Complement or contradiction? CGIAR Program on Participatory Research and Gender Analysis, Working Document No.4, CIAT: Cali. 150pp.
- Deppe, Carol (2000). Breed Your Own Vegetable Varieties. Chelsea Green Publishing.
- Vaschetto, Luis M., ed. (2020). Cereal Genomics. Methods in Molecular Biology. Vol. 2072. doi:10.1007/978-1-4939-9865-4. ISBN 978-1-4939-9864-7. ISSN 1064-3745. S2CID 82398463.
External links
- Plant Breeding and Genomics eXtension Community of Practice – education and training materials for plant breeders and allied professionals
- Plant Breeding Updates
- Hybridization of Crop Plants – large practical reference on plant hybridization
- Infography about the History of Plant Breeding
- Glossary of plant breeding terminology by the Open Plant Breeding Foundation
- National Association of Plant Breeders (NAPB)
- The Global Partnership Initiative for Plant Breeding Capacity Building – GIPB
- FAO/IAEA Programme Mutant Variety Database
- FDA Statement of Policy – Foods Derived from New Plant Varieties
- A Breed Apart: The Plant Breeder's Guide to Preventing Patents through Defensive Publication by Cydnee V. Bence & Emily J. Spiegel, 2019
https://en.wikipedia.org/wiki/Plant_breeding
Breeding in the wild is the natural process of animal reproduction occurring in the natural habitat of a given species. This terminology is distinct from animal husbandry or breeding of species in captivity. Breeding locations are often chosen for very specific requirements of shelter and proximity to food; moreover, the breeding season is a particular time window that has evolved for each species to suit species anatomical, mating-ritual, or climatic and other ecological factors.[1] Many species migrate considerable distances to reach the requisite breeding locations.[2] Certain common characteristics apply to various taxa within the animal kingdom, which traits are often sorted among amphibians, reptiles, mammals, avafauna, arthropods and lower life forms.
Amphibians
For many amphibians, an annual breeding cycle applies, typically regulated by ambient temperature, precipitation, availability of surface water and food supply. This breeding season is accentuated in temperate regions,[3] where prolonged aestivation or hibernation renders many amphibian species inactive for prolonged periods. Breeding habitats are typically ponds and streams.
Mammals
Annual breeding cycles sometimes apply to mammals, with regulating environmental effects including seasonal temperature variation and food availability. Migration patterns of a mammal may sometimes govern breeding times. Mammal breeding in the wild sometimes involves the use of maternity dens for birthing and protection of the young. The polar bear is an example of a mammal who uses a maternity den, whose locations are influenced by migration movements of this species to the seasonal Arctic pack ices. In particular, the polar bears who breed in Wapusk National Park need to migrate to the Hudson Bay pack ice.[4]
Effects of inbreeding in wild populations
Keller and Waller[5] reviewed the effects of inbreeding in wild-populations. Evidence from mammalian and bird populations indicated that inbreeding depression often significantly adversely affects birth weight, reproduction and survival, as well as resistance to environmental stress, disease and predation. Plant studies have shown significant adverse inbreeding effects on seed set, germination, resistance to stress and survival. Inbreeding depression is considered to be largely due to the expression of recessive deleterious alleles.[6]
See also
Line notes
References
- Michael F. Braby (2004) The Complete Field Guide to Butterflies of Australia, Published by CSIRO Publishing, 339 pages ISBN 0-643-09027-4
- Miron L. Heinselman (1996) The Boundary Waters Wilderness ecosystem, University of Minnesota Press, ISBN 0-8166-2805-X
- C. Michael Hogan (2008) Polar Bear: Ursus maritimus, Globaltwitcher.com, ed. Nicklas Stromberg
- William J. Sutherland (1996) Ecological Census Techniques: A Handbook, Cambridge University Press, 336 pages ISBN 0-521-47815-4
https://en.wikipedia.org/wiki/Breeding_in_the_wild
- Breeding refers to nuclear transmutations that produce fuel for further reactions, in a breeder reactor to become fissile material or in a fusion reactor to produce tritium, see Tritium § Lithium
https://en.wikipedia.org/wiki/Breeding
Breeding is sexual reproduction that produces offspring, usually animals or plants. It can only occur between a male and a female animal or plant.
Breeding may refer to:
- Animal husbandry, through selected specimens such as dogs, horses, and rabbits
- Breeding in the wild, the natural process of reproduction in the animal kingdom
- Sexual reproduction of plants
- Plant breeding, through specimens selected by humans for desirable traits
Science
- Breeding refers to nuclear transmutations that produce fuel for further reactions, in a breeder reactor to become fissile material or in a fusion reactor to produce tritium, see Tritium § Lithium
Biology
- Breeding (sex act)
- Breeding back, a breeding effort to re-assemble extinct breed genes
- Breeding pair, bonded animals who cooperate to produce offspring
- Breeding program, a planned breeding of animals or plants
- Breeding season, the period during each year when a species reproduces
- Captive breeding, raising plants or animals in zoos or other controlled conditions
- Cooperative breeding, the raising of the young using non-parental care givers
- Crossbreeding, the process of breeding an animal with purebred parents of two different breeds, varieties, or populations
- Mating
- Preservation breeding, a selection practice to preserve bloodlines
- Selective breeding, an animal selection practice to encourage chosen qualities
- Smart breeding, a plant selection practice to encourage chosen qualities
People
- James Floyd Breeding (1901–1977), U.S. Congressman from Kansas
- Marv Breeding (1934–2006), 1960s U.S. Major League Baseball player
Media
- Breeding (EP), 2007 album by Dirty Little Rabbits
- Breeding Death, 2000 album by Bloodbath
- Breeding the Spawn, 1993 album by Suffocation
- Dust Breeding, 2001 Doctor Who television series audio play
Places
- Breeding, Kentucky, a town in the United States
See also
- Copulation (zoology)
- Good breeding (disambiguation)
- Hybrid (biology), breeding between dissimilar parents
- Inbreeding, breeding between close relatives
- Manners, the unenforced standards of human conduct
- Outbreeding depression, reduced fitness from breeding of unrelated individuals
- Purebred
https://en.wikipedia.org/wiki/Breeding
Preservation breeding is an attempt by many plant and animal breeders to preserve bloodlines of species, either of a rare breed, or of rare pedigrees within a breed.[1][2][3]
Purpose
Preservation breeding can have several purposes:
- Protection of genetic diversity within a species or a breed;[2][4][5]
- Preservation of valuable genetic traits that may not be popular or in fashion in the present, but may be of great value in the future;[1]
- Population or re-population of an area where a species previously existed;
- Support of a wild population that is defective or infected, by breeding healthy individuals and releasing them into the population in order to strengthen the overall health of the population.[6]
Mechanism
Preservation breeding can take the following forms:
- Selective breeding of rare breeds and rare pedigrees, particularly monitoring breeding genetics in small populations to ensure diversity is maintained as much as possible;[2]
- Rare breeds that suffer life-threatening genetic deficiencies can be intentionally cross-bred with other breeds that have the critical gene, in order to preserve the rare breed into the future;[1]
History
The term preservation breeding was first used by notable American Kennel Club Judges Douglas Johnson and Bill Shelton in breeder seminars for dog breeders in the early 2000s. The preservation of dog breeds and the conservancy of canine genetics started gaining more traction in the mid-2010s.
See also
References
- Tessier, Natalie Green. "What can we learn from the devil?". BetterBred.com. Retrieved 2020-04-17.
https://en.wikipedia.org/wiki/Preservation_breeding
| Part of a series on |
| Evolutionary biology |
|---|
 |
|
|
|
|
|
|
|
|
|
|
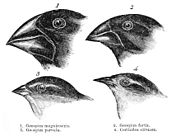
Selective breeding (also called artificial selection) is the process by which humans use animal breeding and plant breeding to selectively develop particular phenotypic traits (characteristics) by choosing which typically animal or plant males and females will sexually reproduce and have offspring together. Domesticated animals are known as breeds, normally bred by a professional breeder, while domesticated plants are known as varieties, cultigens, cultivars, or breeds.[1] Two purebred animals of different breeds produce a crossbreed, and crossbred plants are called hybrids. Flowers, vegetables and fruit-trees may be bred by amateurs and commercial or non-commercial professionals: major crops are usually the provenance of the professionals.
In animal breeding, techniques such as inbreeding, linebreeding, and outcrossing are utilized. In plant breeding, similar methods are used. Charles Darwin discussed how selective breeding had been successful in producing change over time in his 1859 book, On the Origin of Species. Its first chapter discusses selective breeding and domestication of such animals as pigeons, cats, cattle, and dogs. Darwin used artificial selection as an analogy to propose and explain the theory of natural selection but distinguished the latter from the former as a separate process that is non-directed.[2][3][4]
The deliberate exploitation of selective breeding to produce desired results has become very common in agriculture and experimental biology.
Selective breeding can be unintentional, for example, resulting from the process of human cultivation; and it may also produce unintended – desirable or undesirable – results. For example, in some grains, an increase in seed size may have resulted from certain ploughing practices rather than from the intentional selection of larger seeds. Most likely, there has been an interdependence between natural and artificial factors that have resulted in plant domestication.[5]
History
Selective breeding of both plants and animals has been practiced since early prehistory; key species such as wheat, rice, and dogs have been significantly different from their wild ancestors for millennia, and maize, which required especially large changes from teosinte, its wild form, was selectively bred in Mesoamerica. Selective breeding was practiced by the Romans.[6] Treatises as much as 2,000 years old give advice on selecting animals for different purposes, and these ancient works cite still older authorities, such as Mago the Carthaginian.[7] The notion of selective breeding was later expressed by the Persian Muslim polymath Abu Rayhan Biruni in the 11th century. He noted the idea in his book titled India, which included various examples.[8]
The agriculturist selects his corn, letting grow as much as he requires, and tearing out the remainder. The forester leaves those branches which he perceives to be excellent, whilst he cuts away all others. The bees kill those of their kind who only eat, but do not work in their beehive.
— Abu Rayhan Biruni, India
Selective breeding was established as a scientific practice by Robert Bakewell during the British Agricultural Revolution in the 18th century. Arguably, his most important breeding program was with sheep. Using native stock, he was able to quickly select for large, yet fine-boned sheep, with long, lustrous wool. The Lincoln Longwool was improved by Bakewell, and in turn the Lincoln was used to develop the subsequent breed, named the New (or Dishley) Leicester. It was hornless and had a square, meaty body with straight top lines.[9]
These sheep were exported widely, including to Australia and North America, and have contributed to numerous modern breeds, despite the fact that they fell quickly out of favor as market preferences in meat and textiles changed. Bloodlines of these original New Leicesters survive today as the English Leicester (or Leicester Longwool), which is primarily kept for wool production.
Bakewell was also the first to breed cattle to be used primarily for beef. Previously, cattle were first and foremost kept for pulling ploughs as oxen[10][citation needed], but he crossed long-horned heifers and a Westmoreland bull to eventually create the Dishley Longhorn. As more and more farmers followed his lead, farm animals increased dramatically in size and quality. In 1700, the average weight of a bull sold for slaughter was 370 pounds (168 kg). By 1786, that weight had more than doubled to 840 pounds (381 kg). However, after his death, the Dishley Longhorn was replaced with short-horn versions.
He also bred the Improved Black Cart horse, which later became the Shire horse.
Charles Darwin coined the term 'selective breeding'; he was interested in the process as an illustration of his proposed wider process of natural selection. Darwin noted that many domesticated animals and plants had special properties that were developed by intentional animal and plant breeding from individuals that showed desirable characteristics, and discouraging the breeding of individuals with less desirable characteristics.
Darwin used the term "artificial selection" twice in the 1859 first edition of his work On the Origin of Species, in Chapter IV: Natural Selection, and in Chapter VI: Difficulties on Theory:
Slow though the process of selection may be, if feeble man can do much by his powers of artificial selection, I can see no limit to the amount of change, to the beauty and infinite complexity of the co-adaptations between all organic beings, one with another and with their physical conditions of life, which may be effected in the long course of time by nature's power of selection.[11]
— Charles Darwin, On the Origin of Species
We are profoundly ignorant of the causes producing slight and unimportant variations; and we are immediately made conscious of this by reflecting on the differences in the breeds of our domesticated animals in different countries,—more especially in the less civilized countries where there has been but little artificial selection.[12]
— Charles Darwin, On the Origin of Species
Animal breeding
Animals with homogeneous appearance, behavior, and other characteristics are known as particular breeds or pure breeds, and they are bred through culling animals with particular traits and selecting for further breeding those with other traits. Purebred animals have a single, recognizable breed, and purebreds with recorded lineage are called pedigreed. Crossbreeds are a mix of two purebreds, whereas mixed breeds are a mix of several breeds, often unknown. Animal breeding begins with breeding stock, a group of animals used for the purpose of planned breeding. When individuals are looking to breed animals, they look for certain valuable traits in purebred stock for a certain purpose, or may intend to use some type of crossbreeding to produce a new type of stock with different, and, it is presumed, superior abilities in a given area of endeavor. For example, to breed chickens, a breeder typically intends to receive eggs, meat, and new, young birds for further reproduction. Thus, the breeder has to study different breeds and types of chickens and analyze what can be expected from a certain set of characteristics before he or she starts breeding them. Therefore, when purchasing initial breeding stock, the breeder seeks a group of birds that will most closely fit the purpose intended.
Purebred breeding aims to establish and maintain stable traits, that animals will pass to the next generation. By "breeding the best to the best," employing a certain degree of inbreeding, considerable culling, and selection for "superior" qualities, one could develop a bloodline superior in certain respects to the original base stock. Such animals can be recorded with a breed registry, the organization that maintains pedigrees and/or stud books. However, single-trait breeding, breeding for only one trait over all others, can be problematic.[13] In one case mentioned by animal behaviorist Temple Grandin, roosters bred for fast growth or heavy muscles did not know how to perform typical rooster courtship dances, which alienated the roosters from hens and led the roosters to kill the hens after mating with them.[13] A Soviet attempt to breed lab rats with higher intelligence led to cases of neurosis severe enough to make the animals incapable of any problem solving unless drugs like phenazepam were used.[14]
The observable phenomenon of hybrid vigor stands in contrast to the notion of breed purity. However, on the other hand, indiscriminate breeding of crossbred or hybrid animals may also result in degradation of quality. Studies in evolutionary physiology, behavioral genetics, and other areas of organismal biology have also made use of deliberate selective breeding, though longer generation times and greater difficulty in breeding can make these projects challenging in such vertebrates as house mice.[15][16][17]
Plant breeding
Plant breeding has been used for thousands of years, and began with the domestication of wild plants into uniform and predictable agricultural cultigens. High-yielding varieties have been particularly important in agriculture.
Selective plant breeding is also used in research to produce transgenic animals that breed "true" (i.e., are homozygous) for artificially inserted or deleted genes.[18]
Selective breeding in aquaculture
Selective breeding in aquaculture holds high potential for the genetic improvement of fish and shellfish. Unlike terrestrial livestock, the potential benefits of selective breeding in aquaculture were not realized until recently. This is because high mortality led to the selection of only a few broodstock, causing inbreeding depression, which then forced the use of wild broodstock. This was evident in selective breeding programs for growth rate, which resulted in slow growth and high mortality.[19]
Control of the reproduction cycle was one of the main reasons as it is a requisite for selective breeding programs. Artificial reproduction was not achieved because of the difficulties in hatching or feeding some farmed species such as eel and yellowtail farming.[20] A suspected reason associated with the late realisation of success in selective breeding programs in aquaculture was the education of the concerned people – researchers, advisory personnel and fish farmers. The education of fish biologists paid less attention to quantitative genetics and breeding plans.[21]
Another was the failure of documentation of the genetic gains in successive generations. This in turn led to failure in quantifying economic benefits that successful selective breeding programs produce. Documentation of the genetic changes was considered important as they help in fine tuning further selection schemes.[19]
Quality traits in aquaculture
Aquaculture species are reared for particular traits such as growth rate, survival rate, meat quality, resistance to diseases, age at sexual maturation, fecundity, shell traits like shell size, shell colour, etc.
- Growth rate – growth rate is normally measured as either body weight or body length. This trait is of great economic importance for all aquaculture species as faster growth rate speeds up the turnover of production.[21] Improved growth rates show that farmed animals utilize their feed more efficiently through a positive correlated response.[20]
- Survival rate – survival rate may take into account the degrees of resistance to diseases.[20] This may also see the stress response as fish under stress are highly vulnerable to diseases.[21] The stress fish experience could be of biological, chemical or environmental influence.
- Meat quality – the quality of fish is of great economic importance in the market. Fish quality usually takes into account size, meatiness, and percentage of fat, colour of flesh, taste, shape of the body, ideal oil and omega-3 content.[20][22]
- Age at sexual maturation – The age of maturity in aquaculture species is another very important attribute for farmers as during early maturation the species divert all their energy to gonad production affecting growth and meat production and are more susceptible to health problems (Gjerde 1986).
- Fecundity – As the fecundity in fish and shellfish is usually high it is not considered as a major trait for improvement. However, selective breeding practices may consider the size of the egg and correlate it with survival and early growth rate.[20]
Finfish response to selection
Salmonids
Gjedrem (1979) showed that selection of Atlantic salmon (Salmo salar) led to an increase in body weight by 30% per generation. A comparative study on the performance of select Atlantic salmon with wild fish was conducted by AKVAFORSK Genetics Centre in Norway. The traits, for which the selection was done included growth rate, feed consumption, protein retention, energy retention, and feed conversion efficiency. Selected fish had a twice better growth rate, a 40% higher feed intake, and an increased protein and energy retention. This led to an overall 20% better Fed Conversion Efficiency as compared to the wild stock.[23] Atlantic salmon have also been selected for resistance to bacterial and viral diseases. Selection was done to check resistance to Infectious Pancreatic Necrosis Virus (IPNV). The results showed 66.6% mortality for low-resistant species whereas the high-resistant species showed 29.3% mortality compared to wild species.[24]
Rainbow trout (S. gairdneri) was reported to show large improvements in growth rate after 7–10 generations of selection.[25] Kincaid et al. (1977) showed that growth gains by 30% could be achieved by selectively breeding rainbow trout for three generations.[26] A 7% increase in growth was recorded per generation for rainbow trout by Kause et al. (2005).[27]
In Japan, high resistance to IPNV in rainbow trout has been achieved by selectively breeding the stock. Resistant strains were found to have an average mortality of 4.3% whereas 96.1% mortality was observed in a highly sensitive strain.[28]
Coho salmon (Oncorhynchus kisutch) increase in weight was found to be more than 60% after four generations of selective breeding.[29] In Chile, Neira et al. (2006) conducted experiments on early spawning dates in coho salmon. After selectively breeding the fish for four generations, spawning dates were 13–15 days earlier.[30]
Cyprinids
Selective breeding programs for the Common carp (Cyprinus carpio) include improvement in growth, shape and resistance to disease. Experiments carried out in the USSR used crossings of broodstocks to increase genetic diversity and then selected the species for traits like growth rate, exterior traits and viability, and/or adaptation to environmental conditions like variations in temperature. Kirpichnikov et al. (1974)[31] and Babouchkine (1987)[32] selected carp for fast growth and tolerance to cold, the Ropsha carp. The results showed a 30–40% to 77.4% improvement of cold tolerance but did not provide any data for growth rate. An increase in growth rate was observed in the second generation in Vietnam.[33] Moav and Wohlfarth (1976) showed positive results when selecting for slower growth for three generations compared to selecting for faster growth. Schaperclaus (1962) showed resistance to the dropsy disease wherein selected lines suffered low mortality (11.5%) compared to unselected (57%).[34]
Channel Catfish
Growth was seen to increase by 12–20% in selectively bred Iictalurus punctatus.[35] More recently, the response of the Channel Catfish to selection for improved growth rate was found to be approximately 80%, that is, an average of 13% per generation.
Shellfish response to selection
Oysters
Selection for live weight of Pacific oysters showed improvements ranging from 0.4% to 25.6% compared to the wild stock.[36] Sydney-rock oysters (Saccostrea commercialis) showed a 4% increase after one generation and a 15% increase after two generations.[37][38] Chilean oysters (Ostrea chilensis), selected for improvement in live weight and shell length showed a 10–13% gain in one generation. Bonamia ostrea is a protistan parasite that causes catastrophic losses (nearly 98%) in European flat oyster Ostrea edulis L. This protistan parasite is endemic to three oyster-regions in Europe. Selective breeding programs show that O. edulis susceptibility to the infection differs across oyster strains in Europe. A study carried out by Culloty et al. showed that ‘Rossmore' oysters in Cork harbour, Ireland had better resistance compared to other Irish strains. A selective breeding program at Cork harbour uses broodstock from 3– to 4-year-old survivors and is further controlled until a viable percentage reaches market size.[39][40]
Over the years ‘Rossmore' oysters have shown to develop lower prevalence of B. ostreae infection and percentage mortality. Ragone Calvo et al. (2003) selectively bred the eastern oyster, Crassostrea virginica, for resistance against co-occurring parasites Haplosporidium nelson (MSX) and Perkinsus marinus (Dermo). They achieved dual resistance to the disease in four generations of selective breeding. The oysters showed higher growth and survival rates and low susceptibility to the infections. At the end of the experiment, artificially selected C. virginica showed a 34–48% higher survival rate.[41]
Penaeid shrimps
Selection for growth in Penaeid shrimps yielded successful results. A selective breeding program for Litopenaeus stylirostris saw an 18% increase in growth after the fourth generation and 21% growth after the fifth generation.[42] Marsupenaeus japonicas showed a 10.7% increase in growth after the first generation.[43] Argue et al. (2002) conducted a selective breeding program on the Pacific White Shrimp, Litopenaeus vannamei at The Oceanic Institute, Waimanalo, USA from 1995 to 1998. They reported significant responses to selection compared to the unselected control shrimps. After one generation, a 21% increase was observed in growth and 18.4% increase in survival to TSV.[44] The Taura Syndrome Virus (TSV) causes mortalities of 70% or more in shrimps. C.I. Oceanos S.A. in Colombia selected the survivors of the disease from infected ponds and used them as parents for the next generation. They achieved satisfying results in two or three generations wherein survival rates approached levels before the outbreak of the disease.[45] The resulting heavy losses (up to 90%) caused by Infectious hypodermal and haematopoietic necrosis virus (IHHNV) caused a number of shrimp farming industries started to selectively breed shrimps resistant to this disease. Successful outcomes led to development of Super Shrimp, a selected line of L. stylirostris that is resistant to IHHNV infection. Tang et al. (2000) confirmed this by showing no mortalities in IHHNV- challenged Super Shrimp post larvae and juveniles.[46]
Aquatic species versus terrestrial livestock
Selective breeding programs for aquatic species provide better outcomes compared to terrestrial livestock. This higher response to selection of aquatic farmed species can be attributed to the following:
- High fecundity in both sexes fish and shellfish enabling higher selection intensity.
- Large phenotypic and genetic variation in the selected traits.
Selective breeding in aquaculture provide remarkable economic benefits to the industry, the primary one being that it reduces production costs due to faster turnover rates. This is because of faster growth rates, decreased maintenance rates, increased energy and protein retention, and better feed efficiency.[19] Applying such genetic improvement program to aquaculture species will increase productivity to meet the increasing demands of growing populations.
Advantages and disadvantages
Selective breeding is a direct way to determine if a specific trait can evolve in response to selection. A single-generation method of breeding is not as accurate or direct. The process is also more practical and easier to understand than sibling analysis. Selective breeding is better for traits such as physiology and behavior that are hard to measure because it requires fewer individuals to test than single-generation testing.
However, there are disadvantages to this process. Because a single experiment done in selective breeding cannot be used to assess an entire group of genetic variances, individual experiments must be done for every individual trait. Also, because of the necessity of selective breeding experiments to require maintaining the organisms tested in a lab or greenhouse, it is impractical to use this breeding method on many organisms. Controlled mating instances are difficult to carry out in this case and this is a necessary component of selective breeding.[47]
See also
- Animal breeding
- Animal husbandry
- Breed registry
- Breeding back
- Captive breeding
- Culling
- Eugenics
- Experimental evolution
- Gene pool
- Genetic engineering
- Genomics of domestication
- Inbreeding
- Marker-assisted selection
- Mutation breeding
- Natural selection
- Plant breeding
- Serial passage
- Quantitative genetics
- Selection methods in plant breeding based on mode of reproduction
- Smart breeding
References
- Conner, J. K. (2003). "Artificial Selection: A Powerful Tool for Ecologists". Ecology. 84 (7): 1650–1660. doi:10.1890/0012-9658(2003)084[1650:asaptf]2.0.co;2.
Bibliography
- Darwin, Charles (2004). The Origin of Species. London: CRW Publishing Limited. ISBN 978-1-904633-78-5.
Further reading
- FAO. 2007. The Global Plan of Action for Animal Genetic Resources and the Interlaken Declaration. Rome.
- FAO. 2015. The Second Report on the State of the World's Animal Genetic Resources for Food and Agriculture. Rome.
- Gjerdem, B (1986). "Growth and reproduction in fish and shellfish". Aquaculture. 57 (1–4): 37–55. doi:10.1016/0044-8486(86)90179-1.
- Gjedrem, T (1979). "Selection for growth rate and domestication in Atlantic salmon". Zeitschrift für Tierzüchtung und Züchtungsbiologie. 96 (1–4): 56–59. doi:10.1111/j.1439-0388.1979.tb00199.x.
- Gjedrem, T. (1477). "Selective breeding to improve aquaculture production". World Aquaculture. 28: 33–45.
- Purugganan, Michael D.; Fuller, Dorian Q. (2009). "The nature of selection during plant domestication". Nature. 457 (7231): 843–848. Bibcode:2009Natur.457..843P. doi:10.1038/nature07895. PMID 19212403. S2CID 205216444.
- Schäperclaus, W. (1962). Traité de pisciculture en étang. Paris: Vigot Frères.
External links
https://en.wikipedia.org/wiki/Selective_breeding
Good breeding may refer to:
https://en.wikipedia.org/wiki/Good_breeding
In biology, outbreeding depression happens when crosses between two genetically distant groups or populations result in a reduction of fitness.[1] The concept is in contrast to inbreeding depression, although the two effects can occur simultaneously.[2] Outbreeding depression is a risk that sometimes limits the potential for genetic rescue or augmentations.[1] It is considered postzygotic response because outbreeding depression is noted usually in the performance of the progeny.[3]
Outbreeding depression manifests in two ways:
- Generating intermediate genotypes that are less fit than either parental form. For example, selection in one population might favor a large body size, whereas in another population small body size might be more advantageous, while individuals with intermediate body sizes are comparatively disadvantaged in both populations. As another example, in the Tatra Mountains, the introduction of ibex from the Middle East resulted in hybrids which produced calves at the coldest time of the year.[4]
- Breakdown of biochemical or physiological compatibility. Within isolated breeding populations, alleles are selected in the context of the local genetic background. Because the same alleles may have rather different effects in different genetic backgrounds, this can result in different locally coadapted gene complexes. Outcrossing between individuals with differently adapted gene complexes can result in disruption of this selective advantage, resulting in a loss of fitness.
https://en.wikipedia.org/wiki/Outbreeding_depression
Mechanisms for generating outbreeding depression
The different mechanisms of outbreeding depression can operate at the same time. However, determining which mechanism is likely to occur in a particular population can be very difficult.
There are three main mechanisms for generating outbreeding depression:
- Fixed chromosomal differences resulting in the partial or complete sterility of F1 hybrids.[1]
- Adaptive differentiation among populations
- Population bottlenecks and genetic drift
Some mechanisms may not appear until two or more generations later (F2 or greater),[5] when recombination has undermined vitality of positive epistasis. Hybrid vigor in the first generation can, in some circumstances, be strong enough to mask the effects of outbreeding depression. An example of this is that plant breeders will make F1 hybrids from purebred strains, which will improve the uniformity and vigor of the offspring, however the F2 generation are not used for further breeding because of unpredictable phenotypes in their offspring. Unless there is strong selective pressure, outbreeding depression can increase in further generations as coadapted gene complexes are broken apart without the forging of new coadapted gene complexes to take their place. If the outcrossing is limited and populations are large enough, selective pressure acting on each generation can restore fitness. Unless the F1 hybrid generation is sterile or very low fitness, selection will act in each generation using the increased diversity to adapt to the environment.[6] This can lead to recovery in fitness to baseline, and sometimes even greater fitness than original parental types in that environment.[7] However, as the hybrid population will likely to go through a decline in fitness for a few generations, they will need to persist long enough to allow selection to act before they can rebound.[8]
Examples
The first mechanism has the greatest effects on fitness for polyploids, an intermediate effect on translocations, and a modest effect on centric fusions and inversions.[1] Generally this mechanism will be more prevalent in the first generation (F1) after the initial outcrossing when most individuals are made up of the intermediate phenotype.
Examples of the second mechanism include stickleback fish, which developed benthic and limnetic forms when separated. When crosses occurred between the two forms, there were low spawning rates. However, when the same forms mated with each other and no crossing occurred between lakes, the spawning rates were normal. This pattern has also been studied in Drosophila and leaf beetles, where the F1 progeny and later progeny resulted in intermediate fitness between the two parents. This circumstance is more likely to happen and occurs more quickly with selection than genetic drift.[1]
For the third mechanism, examples include poison dart frogs, anole lizards, and cichlid fish. Selection over genetic drift seems to be the dominant mechanism for outbreeding depression.[1]
In plants
For plants, outbreeding depression represents a partial crossing barrier.[3] Unfortunately, outbreeding depression is not understood well in angiosperms. After observing Ipomopsis aggregata over time by crossing plants that were between 10–100m apart, a pattern was noticed that plants that were farther away spatially had a higher likelihood of outbreeding depression.[3] Some general takeaways from this were that spatial patterns of selection on plant genotypes will vary in scale and pattern, and outbreeding depression reflects the genetic constitution of "hybrid" progeny and the environments in which the parents and progeny grow.[3] This means that although outbreeding depression cannot be predicted in angiosperms yet, the environment has a role in it.
See also
References
 This article incorporates public domain material from Michael Lynch. Inbreeding depression and outbreeding depression. National Park Service.
This article incorporates public domain material from Michael Lynch. Inbreeding depression and outbreeding depression. National Park Service.
- Frankham, Ballou, & Briscoe, R., J.D. & D.A. (2002). Introduction to Conservation Genetics. Cambridge. p. 388 ISBN 0521702712
https://en.wikipedia.org/wiki/Outbreeding_depression
Genetic rescue is seen as a mitigation strategy designed to restore genetic diversity and reduce extinction risks in small, isolated and frequently inbred populations.[1] It is largely implemented through translocation, a type of demographic rescue and technical migration that adds individuals to a population to prevent its potential extinction. This demographic rescue may be similar to genetic rescue, as each increase population size and/or fitness. This overlap in meaning has led some researchers to consider a more detailed definition for each type of rescue that details 'assessment and documentation of pre- and post-translocation genetic ancestry'.[1] Not every example of genetic rescue is clearly successful and the current definition of genetic rescue does not mandate that the process result in a 'successful' outcome. Despite an ambiguous definition, genetic rescue is viewed positively, with many perceived successes.[2]
https://en.wikipedia.org/wiki/Genetic_rescue
Evolutionary rescue is a process by which a population—that would have gone extinct in the absence of evolution—persists due to natural selection acting on heritable variation.[1][2] The term was first used in 1995 by Gomulkiewicz and Holt [3] in the context of a sudden environmental change, but the process was studied long before in the context of continuous environmental change[4] and, especially, drug resistance evolution.[5]
https://en.wikipedia.org/wiki/Evolutionary_rescue
Controversy
Genetic rescue can be a controversial tool because it is hard to predict how a population will be affected by a migration event. Genetic rescue has the possibility of actually lowering the fitness of a population by swamping the population or increasing rare deleterious alleles.[10] This instance may simply be termed genetic pollution instead of being referred to genetic rescue. Rescue may also only be a short-term solution, as shown by the case of the Isle Royale Wolves. In that case, genetic rescue of the wolves resulted in a large initial increase in population fitness followed by a large decline in subsequent years.[10] Many conservationists argue that genetic rescue could create unforeseen problems for species at risk, and that it overlooks the underlying problems that push so many species to the brink of extinction, including habitat loss due to human development.[11]
As with the term genetic pollution, 'genetic rescue' has political connotations. Some of the more controversial practices which can be considered genetic rescue include
- De-extinction.[12]
- Genetic engineering to produce plants resistant to pests and disease.[13][14] Although some environmental groups include the use of genetic engineering to save species in their use of genetic pollution.
- Hybridization that increases the viability of endangered populations suffering from inbreeding and genetic drift or saves endlings may be considered part of genetic rescue.[15]
https://en.wikipedia.org/wiki/Genetic_rescue
In population genetics, gene flow (also known as migration and allele flow) is the transfer of genetic material from one population to another. If the rate of gene flow is high enough, then two populations will have equivalent allele frequencies and therefore can be considered a single effective population. It has been shown that it takes only "one migrant per generation" to prevent populations from diverging due to drift.[1] Populations can diverge due to selection even when they are exchanging alleles, if the selection pressure is strong enough.[2][3] Gene flow is an important mechanism for transferring genetic diversity among populations. Migrants change the distribution of genetic diversity among populations, by modifying allele frequencies (the proportion of members carrying a particular variant of a gene). High rates of gene flow can reduce the genetic differentiation between the two groups, increasing homogeneity.[4] For this reason, gene flow has been thought to constrain speciation and prevent range expansion by combining the gene pools of the groups, thus preventing the development of differences in genetic variation that would have led to differentiation and adaptation.[5] In some cases dispersal resulting in gene flow may also result in the addition of novel genetic variants under positive selection to the gene pool of a species or population (adaptive introgression.[6])
https://en.wikipedia.org/wiki/Gene_flow
| Part of a series on |
| Evolutionary biology |
|---|
 |
|
|
|
|
|
|
|
|
|
|
Genetic drift, also known as allelic drift or the Wright effect,[1] is the change in the frequency of an existing gene variant (allele) in a population due to random chance.[2]
Genetic drift may cause gene variants to disappear completely and thereby reduce genetic variation.[3] It can also cause initially rare alleles to become much more frequent and even fixed.
When few copies of an allele exist, the effect of genetic drift is more notable, and when many copies exist, the effect is less notable. In the middle of the 20th century, vigorous debates occurred over the relative importance of natural selection versus neutral processes, including genetic drift. Ronald Fisher, who explained natural selection using Mendelian genetics,[4] held the view that genetic drift plays at most a minor role in evolution, and this remained the dominant view for several decades. In 1968, population geneticist Motoo Kimura rekindled the debate with his neutral theory of molecular evolution, which claims that most instances where a genetic change spreads across a population (although not necessarily changes in phenotypes) are caused by genetic drift acting on neutral mutations.[5][6] In the 1990s, constructive neutral evolution was proposed which seeks to explain how complex systems emerge through neutral transitions.[7][8]
https://en.wikipedia.org/wiki/Genetic_drift
Category:Animal conservation
- Animals by conservation status (17 C, 5 P)
A
- Arthropod conservation (2 C, 5 P)
B
- Bird conservation (10 C, 63 P)
E
- EDGE species (2 C, 123 P)
M
- Mammal conservation (8 C, 26 P)
R
- Reptile conservation (3 C, 4 P)
Pages in category "Animal conservation"
The following 3 pages are in this category, out of 3 total. This list may not reflect recent changes.
https://en.wikipedia.org/wiki/Category:Animal_conservation
De-extinction (also known as resurrection biology, or species revivalism) is the process of generating an organism that either resembles or is an extinct species.[1] There are several ways to carry out the process of de-extinction. Cloning is the most widely proposed method, although genome editing and selective breeding have also been considered. Similar techniques have been applied to certain endangered species, in hopes to boost their genetic diversity. The only method of the three that would provide an animal with the same genetic identity is cloning.[2] There are benefits and malefits to the process of de-extinction ranging from technological advancements to ethical issues.
https://en.wikipedia.org/wiki/De-extinction
Cloning is the process of producing individual organisms with identical genomes, either by natural or artificial means. In nature, some organisms produce clones through asexual reproduction. In the field of biotechnology, cloning is the process of creating cloned organisms of cells and of DNA fragments.
Etymology
Coined by Herbert J. Webber, the term clone derives from the Ancient Greek word κλών (klōn), twig, which is the process whereby a new plant is created from a twig. In botany, the term lusus was used.[1] In horticulture, the spelling clon was used until the early twentieth century; the final e came into use to indicate the vowel is a "long o" instead of a "short o".[2][3] Since the term entered the popular lexicon in a more general context, the spelling clone has been used exclusively.
Natural cloning
This section needs expansion. You can help by adding to it. (December 2022) |
Natural cloning is the production of clones without the involvement of genetic engineering techniques.[4] It may occur accidentally in the case of identical twins, which are formed when a fertilized egg splits, creating two or more embryos that carry almost identical DNA. It may also be part of asexual reproduction, which is a process where a single parent organism produces genetically identical offspring by itself. [5][6]
Cloning is a natural form of reproduction that has allowed life forms to spread for hundreds of millions of years. It is a reproduction method used by plants, fungi, and bacteria, and is also the way that clonal colonies reproduce themselves.[7][8] Examples of these organisms include blueberry plants, Hazel trees, the Pando trees,[9][10] the Kentucky coffeetree, Myrica, and the American sweetgum.
Molecular cloning
Molecular cloning refers to the process of making multiple molecules. Cloning is commonly used to amplify DNA fragments containing whole genes, but it can also be used to amplify any DNA sequence such as promoters, non-coding sequences and randomly fragmented DNA. It is used in a wide array of biological experiments and practical applications ranging from genetic fingerprinting to large scale protein production. Occasionally, the term cloning is misleadingly used to refer to the identification of the chromosomal location of a gene associated with a particular phenotype of interest, such as in positional cloning. In practice, localization of the gene to a chromosome or genomic region does not necessarily enable one to isolate or amplify the relevant genomic sequence. To amplify any DNA sequence in a living organism, that sequence must be linked to an origin of replication, which is a sequence of DNA capable of directing the propagation of itself and any linked sequence. However, a number of other features are needed, and a variety of specialised cloning vectors (small piece of DNA into which a foreign DNA fragment can be inserted) exist that allow protein production, affinity tagging, single-stranded RNA or DNA production and a host of other molecular biology tools.
Cloning of any DNA fragment essentially involves four steps[11]
- fragmentation - breaking apart a strand of DNA
- ligation – gluing together pieces of DNA in a desired sequence
- transfection – inserting the newly formed pieces of DNA into cells
- screening/selection – selecting out the cells that were successfully transfected with the new DNA
Although these steps are invariable among cloning procedures a number of alternative routes can be selected; these are summarized as a cloning strategy.
Initially, the DNA of interest needs to be isolated to provide a DNA segment of suitable size. Subsequently, a ligation procedure is used where the amplified fragment is inserted into a vector (piece of DNA). The vector (which is frequently circular) is linearised using restriction enzymes, and incubated with the fragment of interest under appropriate conditions with an enzyme called DNA ligase. Following ligation, the vector with the insert of interest is transfected into cells. A number of alternative techniques are available, such as chemical sensitisation of cells, electroporation, optical injection and biolistics. Finally, the transfected cells are cultured. As the aforementioned procedures are of particularly low efficiency, there is a need to identify the cells that have been successfully transfected with the vector construct containing the desired insertion sequence in the required orientation. Modern cloning vectors include selectable antibiotic resistance markers, which allow only cells in which the vector has been transfected, to grow. Additionally, the cloning vectors may contain colour selection markers, which provide blue/white screening (alpha-factor complementation) on X-gal medium. Nevertheless, these selection steps do not absolutely guarantee that the DNA insert is present in the cells obtained. Further investigation of the resulting colonies must be required to confirm that cloning was successful. This may be accomplished by means of PCR, restriction fragment analysis and/or DNA sequencing.
Cell cloning
Cloning unicellular organisms
Cloning a cell means to derive a population of cells from a single cell. In the case of unicellular organisms such as bacteria and yeast, this process is remarkably simple and essentially only requires the inoculation of the appropriate medium. However, in the case of cell cultures from multi-cellular organisms, cell cloning is an arduous task as these cells will not readily grow in standard media.
A useful tissue culture technique used to clone distinct lineages of cell lines involves the use of cloning rings (cylinders).[12] In this technique a single-cell suspension of cells that have been exposed to a mutagenic agent or drug used to drive selection is plated at high dilution to create isolated colonies, each arising from a single and potentially clonal distinct cell. At an early growth stage when colonies consist of only a few cells, sterile polystyrene rings (cloning rings), which have been dipped in grease, are placed over an individual colony and a small amount of trypsin is added. Cloned cells are collected from inside the ring and transferred to a new vessel for further growth.
Cloning stem cells
Somatic-cell nuclear transfer, popularly known as SCNT, can also be used to create embryos for research or therapeutic purposes. The most likely purpose for this is to produce embryos for use in stem cell research. This process is also called "research cloning" or "therapeutic cloning". The goal is not to create cloned human beings (called "reproductive cloning"), but rather to harvest stem cells that can be used to study human development and to potentially treat disease. While a clonal human blastocyst has been created, stem cell lines are yet to be isolated from a clonal source.[13]
Therapeutic cloning is achieved by creating embryonic stem cells in the hopes of treating diseases such as diabetes and Alzheimer's. The process begins by removing the nucleus (containing the DNA) from an egg cell and inserting a nucleus from the adult cell to be cloned.[14] In the case of someone with Alzheimer's disease, the nucleus from a skin cell of that patient is placed into an empty egg. The reprogrammed cell begins to develop into an embryo because the egg reacts with the transferred nucleus. The embryo will become genetically identical to the patient.[14] The embryo will then form a blastocyst which has the potential to form/become any cell in the body.[15]
The reason why SCNT is used for cloning is because somatic cells can be easily acquired and cultured in the lab. This process can either add or delete specific genomes of farm animals. A key point to remember is that cloning is achieved when the oocyte maintains its normal functions and instead of using sperm and egg genomes to replicate, the donor's somatic cell nucleus is inserted into the oocyte.[16] The oocyte will react to the somatic cell nucleus, the same way it would to a sperm cell's nucleus.[16]
The process of cloning a particular farm animal using SCNT is relatively the same for all animals. The first step is to collect the somatic cells from the animal that will be cloned. The somatic cells could be used immediately or stored in the laboratory for later use.[16] The hardest part of SCNT is removing maternal DNA from an oocyte at metaphase II. Once this has been done, the somatic nucleus can be inserted into an egg cytoplasm.[16] This creates a one-cell embryo. The grouped somatic cell and egg cytoplasm are then introduced to an electrical current.[16] This energy will hopefully allow the cloned embryo to begin development. The successfully developed embryos are then placed in surrogate recipients, such as a cow or sheep in the case of farm animals.[16]
SCNT is seen as a good method for producing agriculture animals for food consumption. It successfully cloned sheep, cattle, goats, and pigs. Another benefit is SCNT is seen as a solution to clone endangered species that are on the verge of going extinct.[16] However, stresses placed on both the egg cell and the introduced nucleus can be enormous, which led to a high loss in resulting cells in early research. For example, the cloned sheep Dolly was born after 277 eggs were used for SCNT, which created 29 viable embryos. Only three of these embryos survived until birth, and only one survived to adulthood.[17] As the procedure could not be automated, and had to be performed manually under a microscope, SCNT was very resource intensive. The biochemistry involved in reprogramming the differentiated somatic cell nucleus and activating the recipient egg was also far from being well understood. However, by 2014 researchers were reporting cloning success rates of seven to eight out of ten[18] and in 2016, a Korean Company Sooam Biotech was reported to be producing 500 cloned embryos per day.[19]
In SCNT, not all of the donor cell's genetic information is transferred, as the donor cell's mitochondria that contain their own mitochondrial DNA are left behind. The resulting hybrid cells retain those mitochondrial structures which originally belonged to the egg. As a consequence, clones such as Dolly that are born from SCNT are not perfect copies of the donor of the nucleus.
Organism cloning
Organism cloning (also called reproductive cloning) refers to the procedure of creating a new multicellular organism, genetically identical to another. In essence this form of cloning is an asexual method of reproduction, where fertilization or inter-gamete contact does not take place. Asexual reproduction is a naturally occurring phenomenon in many species, including most plants and some insects. Scientists have made some major achievements with cloning, including the asexual reproduction of sheep and cows. There is a lot of ethical debate over whether or not cloning should be used. However, cloning, or asexual propagation,[20] has been common practice in the horticultural world for hundreds of years.
Horticultural
The term clone is used in horticulture to refer to descendants of a single plant which were produced by vegetative reproduction or apomixis. Many horticultural plant cultivars are clones, having been derived from a single individual, multiplied by some process other than sexual reproduction.[21] As an example, some European cultivars of grapes represent clones that have been propagated for over two millennia. Other examples are potato and banana.[22]
Grafting can be regarded as cloning, since all the shoots and branches coming from the graft are genetically a clone of a single individual, but this particular kind of cloning has not come under ethical scrutiny and is generally treated as an entirely different kind of operation.
Many trees, shrubs, vines, ferns and other herbaceous perennials form clonal colonies naturally. Parts of an individual plant may become detached by fragmentation and grow on to become separate clonal individuals. A common example is in the vegetative reproduction of moss and liverwort gametophyte clones by means of gemmae. Some vascular plants e.g. dandelion and certain viviparous grasses also form seeds asexually, termed apomixis, resulting in clonal populations of genetically identical individuals.
Parthenogenesis
Clonal derivation exists in nature in some animal species and is referred to as parthenogenesis (reproduction of an organism by itself without a mate). This is an asexual form of reproduction that is only found in females of some insects, crustaceans, nematodes,[23] fish (for example the hammerhead shark[24]), Cape honeybees,[25] and lizards including the Komodo dragon[24] and several whiptails. The growth and development occurs without fertilization by a male. In plants, parthenogenesis means the development of an embryo from an unfertilized egg cell, and is a component process of apomixis. In species that use the XY sex-determination system, the offspring will always be female. An example is the little fire ant (Wasmannia auropunctata), which is native to Central and South America but has spread throughout many tropical environments.
Artificial cloning of organisms
Artificial cloning of organisms may also be called reproductive cloning.
First steps
Hans Spemann, a German embryologist was awarded a Nobel Prize in Physiology or Medicine in 1935 for his discovery of the effect now known as embryonic induction, exercised by various parts of the embryo, that directs the development of groups of cells into particular tissues and organs. In 1924 he and his student, Hilde Mangold, were the first to perform somatic-cell nuclear transfer using amphibian embryos – one of the first steps towards cloning.[26]
Methods
Reproductive cloning generally uses "somatic cell nuclear transfer" (SCNT) to create animals that are genetically identical. This process entails the transfer of a nucleus from a donor adult cell (somatic cell) to an egg from which the nucleus has been removed, or to a cell from a blastocyst from which the nucleus has been removed.[27] If the egg begins to divide normally it is transferred into the uterus of the surrogate mother. Such clones are not strictly identical since the somatic cells may contain mutations in their nuclear DNA. Additionally, the mitochondria in the cytoplasm also contains DNA and during SCNT this mitochondrial DNA is wholly from the cytoplasmic donor's egg, thus the mitochondrial genome is not the same as that of the nucleus donor cell from which it was produced. This may have important implications for cross-species nuclear transfer in which nuclear-mitochondrial incompatibilities may lead to death.
Artificial embryo splitting or embryo twinning, a technique that creates monozygotic twins from a single embryo, is not considered in the same fashion as other methods of cloning. During that procedure, a donor embryo is split in two distinct embryos, that can then be transferred via embryo transfer. It is optimally performed at the 6- to 8-cell stage, where it can be used as an expansion of IVF to increase the number of available embryos.[28] If both embryos are successful, it gives rise to monozygotic (identical) twins.
Dolly the sheep
Dolly, a Finn-Dorset ewe, was the first mammal to have been successfully cloned from an adult somatic cell. Dolly was formed by taking a cell from the udder of her 6-year-old biological mother.[29] Dolly's embryo was created by taking the cell and inserting it into a sheep ovum. It took 435 attempts before an embryo was successful.[30] The embryo was then placed inside a female sheep that went through a normal pregnancy.[31] She was cloned at the Roslin Institute in Scotland by British scientists Sir Ian Wilmut and Keith Campbell and lived there from her birth in 1996 until her death in 2003 when she was six. She was born on 5 July 1996 but not announced to the world until 22 February 1997.[32] Her stuffed remains were placed at Edinburgh's Royal Museum, part of the National Museums of Scotland.[33]
Dolly was publicly significant because the effort showed that genetic material from a specific adult cell, designed to express only a distinct subset of its genes, can be redesigned to grow an entirely new organism. Before this demonstration, it had been shown by John Gurdon that nuclei from differentiated cells could give rise to an entire organism after transplantation into an enucleated egg.[34] However, this concept was not yet demonstrated in a mammalian system.
The first mammalian cloning (resulting in Dolly) had a success rate of 29 embryos per 277 fertilized eggs, which produced three lambs at birth, one of which lived. In a bovine experiment involving 70 cloned calves, one-third of the calves died quite young. The first successfully cloned horse, Prometea, took 814 attempts. Notably, although the first clones were frogs, no adult cloned frog has yet been produced from a somatic adult nucleus donor cell.[35]
There were early claims that Dolly had pathologies resembling accelerated aging. Scientists speculated that Dolly's death in 2003 was related to the shortening of telomeres, DNA-protein complexes that protect the end of linear chromosomes. However, other researchers, including Ian Wilmut who led the team that successfully cloned Dolly, argue that Dolly's early death due to respiratory infection was unrelated to problems with the cloning process. This idea that the nuclei have not irreversibly aged was shown in 2013 to be true for mice.[36]
Dolly was named after performer Dolly Parton because the cells cloned to make her were from a mammary gland cell, and Parton is known for her ample cleavage.[37]
Species cloned and applications
This section needs expansion. You can help by adding to it. (March 2023) |
The modern cloning techniques involving nuclear transfer have been successfully performed on several species. Notable experiments include:
- Tadpole: (1952) Robert Briggs and Thomas J. King had successfully cloned northern leopard frogs: thirty-five complete embryos and twenty-seven tadpoles from one-hundred and four successful nuclear transfers.[38][39]
- Carp: (1963) In China, embryologist Tong Dizhou produced the world's first cloned fish by inserting the DNA from a cell of a male carp into an egg from a female carp. He published the findings in a Chinese science journal.[40]
- Zebrafish: The first vertebrate cloned (1981) by George Streisinger[41]
- Sheep: Marked the first mammal being cloned (1984) from early embryonic cells by Steen Willadsen. Megan and Morag[42] cloned from differentiated embryonic cells in June 1995 and Dolly from a somatic cell in 1996.[43][40]
- Mice: (1986) A mouse was successfully cloned from an early embryonic cell. Soviet scientists Chaylakhyan, Veprencev, Sviridova, and Nikitin had the mouse "Masha" cloned. Research was published in the magazine Biofizika volume ХХХII, issue 5 of 1987.[clarification needed][44][45][needs update]
- Rhesus monkey: Tetra (January 2000) from embryo splitting and not nuclear transfer. More akin to artificial formation of twins.[46][47]
- Pig: the first cloned pigs (March 2000).[48] By 2014, BGI in China was producing 500 cloned pigs a year to test new medicines.[49]
- Gaur: (2001) was the first endangered species cloned.[50]
- Cattle:
- Alpha and Beta (males, 2001) and (2005), Brazil[51]
- In 2023, Chinese scientists reported the cloning of three supercows with a milk productivity "nearly 1.7 times the amount of milk an average cow in the United States produced in 2021" and a plan for 1,000 of such super cows in the near-term. According to a news report "[i]n many countries, including the United States, farmers breed clones with conventional animals to add desirable traits, such as high milk production or disease resistance, into the gene pool".[clarification needed][when?][52]
- Cat: CopyCat "CC" (female, late 2001), Little Nicky, 2004, was the first cat cloned for commercial reasons[53]
- Rat: Ralph, the first cloned rat (2003)[54]
- Mule: Idaho Gem, a john mule born 4 May 2003, was the first horse-family clone.[55]
- Horse: Prometea, a Haflinger female born 28 May 2003, was the first horse clone.[56]
- Przewalksi's Horse: An ongoing cloning program by the San Diego Zoo Wildlife Alliance and Revive & Restore attempts to reintroduce genetic diversity to this endangered species.[57]
- Dog:
- Snuppy, a male Afghan hound was the first cloned dog (2005).[60] In 2017, the world's first gene-editing clone dog, Apple, was created by Sinogene Biotechnology.[61] Sooam Biotech, South Korea, was reported in 2015 to have cloned 700 dogs to date for their owners, including two Yakutian Laika hunting dogs, which are seriously endangered due to crossbreeding.[62]
- Cloning of super sniffer dogs was reported in 2011, four years afterwards when the dogs started working.[63] Cloning of a successful rescue dog was also reported in 2009[64] and of a similar police dog in 2019.[65] Cancer-sniffing dogs have also been cloned. A review concluded that "qualified elite working dogs can be produced by cloning a working dog that exhibits both an appropriate temperament and good health."[66]
- Wolf: Snuwolf and Snuwolffy, the first two cloned female wolves (2005).[67]
- Water buffalo: Samrupa was the first cloned water buffalo. It was born on 6 February 2009, at India's Karnal National Diary Research Institute but died five days later due to lung infection.[68]
- Pyrenean ibex (2009) was the first extinct animal to be cloned back to life; the clone lived for seven minutes before dying of lung defects.[69][70]
- Camel: (2009) Injaz, was the first cloned camel.[71]
- Pashmina goat: (2012) Noori, is the first cloned pashmina goat. Scientists at the faculty of veterinary sciences and animal husbandry of Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir successfully cloned the first Pashmina goat (Noori) using the advanced reproductive techniques under the leadership of Riaz Ahmad Shah.[72]
- Goat: (2001) Scientists of Northwest A&F University successfully cloned the first goat which use the adult female cell.[73]
- Gastric brooding frog: (2013) The gastric brooding frog, Rheobatrachus silus, thought to have been extinct since 1983 was cloned in Australia, although the embryos died after a few days.[74]
- Macaque monkey: (2017) First successful cloning of a primate species using nuclear transfer, with the birth of two live clones named Zhong Zhong and Hua Hua. Conducted in China in 2017, and reported in January 2018.[75][76][77][78] In January 2019, scientists in China reported the creation of five identical cloned gene-edited monkeys, using the same cloning technique that was used with Zhong Zhong and Hua Hua and Dolly the sheep, and the gene-editing Crispr-Cas9 technique allegedly used by He Jiankui in creating the first ever gene-modified human babies Lulu and Nana. The monkey clones were made to study several medical diseases.[79][80]
- Black-footed ferret: (2020) A team of scientists cloned a female named Willa, who died in the mid-1980s and left no living descendants. Her clone, a female named Elizabeth Ann, was born on 10 December. Scientists hope that the contribution of this individual will alleviate the effects of inbreeding and help black-footed ferrets better cope with plague. Experts estimate that this female's genome contains three times as much genetic diversity as any of the modern black-footed ferrets.[81]
- First artificial parthenogenesis in mammals: (2022) Viable mice offspring was born from unfertilized eggs via targeted DNA methylation editing of seven imprinting control regions.[82]
Human cloning
Human cloning is the creation of a genetically identical copy of a human. The term is generally used to refer to artificial human cloning, which is the reproduction of human cells and tissues. It does not refer to the natural conception and delivery of identical twins. The possibility of human cloning has raised controversies. These ethical concerns have prompted several nations to pass legislation regarding human cloning and its legality. As of right now, scientists have no intention of trying to clone people and they believe their results should spark a wider discussion about the laws and regulations the world needs to regulate cloning.[83]
Two commonly discussed types of theoretical human cloning are therapeutic cloning and reproductive cloning. Therapeutic cloning would involve cloning cells from a human for use in medicine and transplants, and is an active area of research, but is not in medical practice anywhere in the world, as of 2021. Two common methods of therapeutic cloning that are being researched are somatic-cell nuclear transfer and, more recently, pluripotent stem cell induction. Reproductive cloning would involve making an entire cloned human, instead of just specific cells or tissues.[84]
Ethical issues of cloning
There are a variety of ethical positions regarding the possibilities of cloning, especially human cloning. While many of these views are religious in origin, the questions raised by cloning are faced by secular perspectives as well. Perspectives on human cloning are theoretical, as human therapeutic and reproductive cloning are not commercially used; animals are currently cloned in laboratories and in livestock production.
Advocates support development of therapeutic cloning to generate tissues and whole organs to treat patients who otherwise cannot obtain transplants,[85] to avoid the need for immunosuppressive drugs,[84] and to stave off the effects of aging.[86] Advocates for reproductive cloning believe that parents who cannot otherwise procreate should have access to the technology.[87]
Opponents of cloning have concerns that technology is not yet developed enough to be safe[88] and that it could be prone to abuse (leading to the generation of humans from whom organs and tissues would be harvested),[89][90] as well as concerns about how cloned individuals could integrate with families and with society at large.[91][92] Cloning humans could lead to serious violations of human rights.[93]
Religious groups are divided, with some opposing the technology as usurping "God's place" and, to the extent embryos are used, destroying a human life; others support therapeutic cloning's potential life-saving benefits.[94][95] There is at least one religion, Raëlism, in which cloning plays a major role.[96][97][98]
Contemporary work on this topic is concerned with the ethics, adequate regulation and issues of any cloning carried out by humans, not potentially by extraterrestrials (including in the future), and largely also not replication – also described as mind cloning[99][100][101][102] – of potential whole brain emulations.
Cloning of animals is opposed by animal-groups due to the number of cloned animals that suffer from malformations before they die, and while food from cloned animals has been approved as safe by the US FDA,[103][104] its use is opposed by groups concerned about food safety.[105][106]
In practical terms, the inclusion of "licensing requirements for embryo research projects and fertility clinics, restrictions on the commodification of eggs and sperm, and measures to prevent proprietary interests from monopolizing access to stem cell lines" in international cloning regulations has been proposed, albeit e.g. effective oversight mechanisms or cloning requirements have not been described.[107]
Cloning extinct and endangered species
Cloning, or more precisely, the reconstruction of functional DNA from extinct species has, for decades, been a dream. Possible implications of this were dramatized in the 1984 novel Carnosaur and the 1990 novel Jurassic Park.[108][109] The best current cloning techniques have an average success rate of 9.4 percent[110] (and as high as 25 percent[36]) when working with familiar species such as mice,[note 1] while cloning wild animals is usually less than 1 percent successful.[113]
Conservation cloning
Several tissue banks have come into existence, including the "Frozen zoo" at the San Diego Zoo, to store frozen tissue from the world's rarest and most endangered species.[108][114][115][116] This is also referred to as "Conservation cloning".[117][118]
Engineers have proposed a "lunar ark" in 2021 – storing millions of seed, spore, sperm and egg samples from Earth's contemporary species in a network of lava tubes on the Moon as a genetic backup.[119][120][121] Similar proposals have been made since at least 2008.[122] These also include sending human customer DNA,[123] and a proposal for "a lunar backup record of humanity" that includes genetic information by Avi Loeb et al.[124]
Scientists at the University of Newcastle and University of New South Wales announced in March 2013 that the very recently extinct gastric-brooding frog would be the subject of a cloning attempt to resurrect the species.[125]
Many such "De-extinction" projects are being championed by the non-profit Revive & Restore.[126]
De-extinction
One of the most anticipated targets for cloning was once the woolly mammoth, but attempts to extract DNA from frozen mammoths have been unsuccessful, though a joint Russo-Japanese team is currently working toward this goal.[when?] In January 2011, it was reported by Yomiuri Shimbun that a team of scientists headed by Akira Iritani of Kyoto University had built upon research by Dr. Wakayama, saying that they will extract DNA from a mammoth carcass that had been preserved in a Russian laboratory and insert it into the egg cells of an Asian elephant in hopes of producing a mammoth embryo. The researchers said they hoped to produce a baby mammoth within six years.[127][128] It was noted, however that the result, if possible, would be an elephant-mammoth hybrid rather than a true mammoth.[129] Another problem is the survival of the reconstructed mammoth: ruminants rely on a symbiosis with specific microbiota in their stomachs for digestion.[129]
In 2022, scientists showed major limitations and the scale of challenge of genetic-editing-based de-extinction, suggesting resources spent on more comprehensive de-extinction projects such as of the woolly mammoth may currently not be well allocated and substantially limited. Their analyses "show that even when the extremely high-quality Norway brown rat (R. norvegicus) is used as a reference, nearly 5% of the genome sequence is unrecoverable, with 1,661 genes recovered at lower than 90% completeness, and 26 completely absent", complicated further by that "distribution of regions affected is not random, but for example, if 90% completeness is used as the cutoff, genes related to immune response and olfaction are excessively affected" due to which "a reconstructed Christmas Island rat would lack attributes likely critical to surviving in its natural or natural-like environment".[130]
In a 2021 online session of the Russian Geographical Society, Russia's defense minister Sergei Shoigu mentioned using the DNA of 3,000-year-old Scythian warriors to potentially bring them back to life. The idea was described as absurd at least at this point in news reports and it was noted that Scythians likely weren't skilled warriors by default.[131][132][133]
The idea of cloning Neanderthals or bringing them back to life in general is controversial but some scientists have stated that it may be possible in the future and have outlined several issues or problems with such as well as broad rationales for doing so.[134][135][136][137][138][139]
Unsuccessful attempts
In 2001, a cow named Bessie gave birth to a cloned Asian gaur, an endangered species, but the calf died after two days. In 2003, a banteng was successfully cloned, followed by three African wildcats from a thawed frozen embryo. These successes provided hope that similar techniques (using surrogate mothers of another species) might be used to clone extinct species. Anticipating this possibility, tissue samples from the last bucardo (Pyrenean ibex) were frozen in liquid nitrogen immediately after it died in 2000. Researchers are also considering cloning endangered species such as the Giant panda and Cheetah.[140][141][142][143]
In 2002, geneticists at the Australian Museum announced that they had replicated DNA of the thylacine (Tasmanian tiger), at the time extinct for about 65 years, using polymerase chain reaction.[144] However, on 15 February 2005 the museum announced that it was stopping the project after tests showed the specimens' DNA had been too badly degraded by the (ethanol) preservative. On 15 May 2005 it was announced that the thylacine project would be revived, with new participation from researchers in New South Wales and Victoria.[145]
In 2003, for the first time, an extinct animal, the Pyrenean ibex mentioned above was cloned, at the Centre of Food Technology and Research of Aragon, using the preserved frozen cell nucleus of the skin samples from 2001 and domestic goat egg-cells. The ibex died shortly after birth due to physical defects in its lungs.[146]
Lifespan
After an eight-year project involving the use of a pioneering cloning technique, Japanese researchers created 25 generations of healthy cloned mice with normal lifespans, demonstrating that clones are not intrinsically shorter-lived than naturally born animals.[36][147] Other sources have noted that the offspring of clones tend to be healthier than the original clones and indistinguishable from animals produced naturally.[148]
Some posited that Dolly the sheep may have aged more quickly than naturally born animals, as she died relatively early for a sheep at the age of six. Ultimately, her death was attributed to a respiratory illness, and the "advanced aging" theory is disputed.[149][dubious ]
A detailed study released in 2016 and less detailed studies by others suggest that once cloned animals get past the first month or two of life they are generally healthy. However, early pregnancy loss and neonatal losses are still greater with cloning than natural conception or assisted reproduction (IVF). Current research is attempting to overcome these problems.[37]
In popular culture
Discussion of cloning in the popular media often presents the subject negatively. In an article in the 8 November 1993 article of Time, cloning was portrayed in a negative way, modifying Michelangelo's Creation of Adam to depict Adam with five identical hands.[150] Newsweek's 10 March 1997 issue also critiqued the ethics of human cloning, and included a graphic depicting identical babies in beakers.[151]
The concept of cloning, particularly human cloning, has featured a wide variety of science fiction works. An early fictional depiction of cloning is Bokanovsky's Process which features in Aldous Huxley's 1931 dystopian novel Brave New World. The process is applied to fertilized human eggs in vitro, causing them to split into identical genetic copies of the original.[152][153] Following renewed interest in cloning in the 1950s, the subject was explored further in works such as Poul Anderson's 1953 story UN-Man, which describes a technology called "exogenesis", and Gordon Rattray Taylor's book The Biological Time Bomb, which popularised the term "cloning" in 1963.[154]
Cloning is a recurring theme in a number of contemporary science fiction films, ranging from action films such as Anna to the Infinite Power, The Boys from Brazil, Jurassic Park (1993), Alien Resurrection (1997), The 6th Day (2000), Resident Evil (2002), Star Wars: Episode II – Attack of the Clones (2002), The Island (2005) and Moon (2009) to comedies such as Woody Allen's 1973 film Sleeper.[155]
The process of cloning is represented variously in fiction. Many works depict the artificial creation of humans by a method of growing cells from a tissue or DNA sample; the replication may be instantaneous, or take place through slow growth of human embryos in artificial wombs. In the long-running British television series Doctor Who, the Fourth Doctor and his companion Leela were cloned in a matter of seconds from DNA samples ("The Invisible Enemy", 1977) and then – in an apparent homage to the 1966 film Fantastic Voyage – shrunk to microscopic size to enter the Doctor's body to combat an alien virus. The clones in this story are short-lived, and can only survive a matter of minutes before they expire.[156] Science fiction films such as The Matrix and Star Wars: Episode II – Attack of the Clones have featured scenes of human foetuses being cultured on an industrial scale in mechanical tanks.[157]
Cloning humans from body parts is also a common theme in science fiction. Cloning features strongly among the science fiction conventions parodied in Woody Allen's Sleeper, the plot of which centres around an attempt to clone an assassinated dictator from his disembodied nose.[158] In the 2008 Doctor Who story "Journey's End", a duplicate version of the Tenth Doctor spontaneously grows from his severed hand, which had been cut off in a sword fight during an earlier episode.[159]
After the death of her beloved 14-year-old Coton de Tulear named Samantha in late 2017, Barbra Streisand announced that she had cloned the dog, and was now "waiting for [the two cloned pups] to get older so [she] can see if they have [Samantha's] brown eyes and her seriousness".[160] The operation cost $50,000 through the pet cloning company ViaGen.[161]
Cloning and identity
Science fiction has used cloning, most commonly and specifically human cloning, to raise the controversial questions of identity.[162][163] A Number is a 2002 play by English playwright Caryl Churchill which addresses the subject of human cloning and identity, especially nature and nurture. The story, set in the near future, is structured around the conflict between a father (Salter) and his sons (Bernard 1, Bernard 2, and Michael Black) – two of whom are clones of the first one. A Number was adapted by Caryl Churchill for television, in a co-production between the BBC and HBO Films.[164]
In 2012, a Japanese television series named "Bunshin" was created. The story's main character, Mariko, is a woman studying child welfare in Hokkaido. She grew up always doubtful about the love from her mother, who looked nothing like her and who died nine years before. One day, she finds some of her mother's belongings at a relative's house, and heads to Tokyo to seek out the truth behind her birth. She later discovered that she was a clone.[165]
In the 2013 television series Orphan Black, cloning is used as a scientific study on the behavioral adaptation of the clones.[166] In a similar vein, the book The Double by Nobel Prize winner José Saramago explores the emotional experience of a man who discovers that he is a clone.[167]
Cloning as resurrection
Cloning has been used in fiction as a way of recreating historical figures. In the 1976 Ira Levin novel The Boys from Brazil and its 1978 film adaptation, Josef Mengele uses cloning to create copies of Adolf Hitler.[168]
In Michael Crichton's 1990 novel Jurassic Park, which spawned a series of Jurassic Park feature films, the bioengineering company InGen develops a technique to resurrect extinct species of dinosaurs by creating cloned creatures using DNA extracted from fossils. The cloned dinosaurs are used to populate the Jurassic Park wildlife park for the entertainment of visitors. The scheme goes disastrously wrong when the dinosaurs escape their enclosures. Despite being selectively cloned as females to prevent them from breeding, the dinosaurs develop the ability to reproduce through parthenogenesis.[169]
Cloning for warfare
The use of cloning for military purposes has also been explored in several fictional works. In Doctor Who, an alien race of armour-clad, warlike beings called Sontarans was introduced in the 1973 serial "The Time Warrior". Sontarans are depicted as squat, bald creatures who have been genetically engineered for combat. Their weak spot is a "probic vent", a small socket at the back of their neck which is associated with the cloning process.[170] The concept of cloned soldiers being bred for combat was revisited in "The Doctor's Daughter" (2008), when the Doctor's DNA is used to create a female warrior called Jenny.[171]
The 1977 film Star Wars was set against the backdrop of a historical conflict called the Clone Wars. The events of this war were not fully explored until the prequel films Attack of the Clones (2002) and Revenge of the Sith (2005), which depict a space war waged by a massive army of heavily armoured clone troopers that leads to the foundation of the Galactic Empire. Cloned soldiers are "manufactured" on an industrial scale, genetically conditioned for obedience and combat effectiveness. It is also revealed that the popular character Boba Fett originated as a clone of Jango Fett, a mercenary who served as the genetic template for the clone troopers.[172][173]
Cloning for exploitation
A recurring sub-theme of cloning fiction is the use of clones as a supply of organs for transplantation. The 2005 Kazuo Ishiguro novel Never Let Me Go and the 2010 film adaption[174] are set in an alternate history in which cloned humans are created for the sole purpose of providing organ donations to naturally born humans, despite the fact that they are fully sentient and self-aware. The 2005 film The Island[175] revolves around a similar plot, with the exception that the clones are unaware of the reason for their existence.
The exploitation of human clones for dangerous and undesirable work was examined in the 2009 British science fiction film Moon.[176] In the futuristic novel Cloud Atlas and subsequent film, one of the story lines focuses on a genetically engineered fabricant clone named Sonmi~451, one of millions raised in an artificial "wombtank", destined to serve from birth. She is one of thousands created for manual and emotional labor; Sonmi herself works as a server in a restaurant. She later discovers that the sole source of food for clones, called 'Soap', is manufactured from the clones themselves.[177]
In the film Us, at some point prior to the 1980s, the US Government creates clones of every citizen of the United States with the intention of using them to control their original counterparts, akin to voodoo dolls. This fails, as they were able to copy bodies, but unable to copy the souls of those they cloned. The project is abandoned and the clones are trapped exactly mirroring their above-ground counterparts' actions for generations. In the present day, the clones launch a surprise attack and manage to complete a mass-genocide of their unaware counterparts.[178][179]
See also
Notes
References
- News article about the study: "A single honeybee has cloned itself hundreds of millions of times". New Scientist. Retrieved 8 March 2023.
- News article about the study: Magazine, Smithsonian; Osborne, Margaret. "Mice Birthed From Unfertilized Eggs for the First Time". Smithsonian Magazine. Retrieved 18 April 2022.
- Article about the study: Tognetti, Laurence. "The moon is the perfect spot for humanity's offsite backup". Universe Today via phys.org. Retrieved 8 March 2023.
- News report on the study: Ahmed, Issam. "Forget mammoths, study shows how to resurrect Christmas Island rats". phys.org. Retrieved 19 April 2022.
- Jason Spiegel (23 March 2019). "'Us' Ending Explained: Could There Be A Sequel?". The Hollywood Reporter. Retrieved 11 July 2019.
Further reading
- Guo, Owen. "World's Biggest Animal Cloning Center Set for '16 in a Skeptical China". The New York Times, 26 November 2015
- Lerner, K. Lee. "Animal cloning". The Gale Encyclopedia of Science, edited by K. Lee Lerner and Brenda Wilmoth Lerner, 5th ed., Gale, 2014. Science in Context, link[permanent dead link]
- Dutchen, Stephanie (11 July 2018). "Rise of the Clones". Harvard Medical School.
External links
| Library resources about Cloning |
- "Cloning". Internet Encyclopedia of Philosophy.
- Cloning Fact Sheet from Human Genome Project Information website.
- 'Cloning' Freeview video by the Vega Science Trust and the BBC/OU
- Cloning in Focus, an accessible and comprehensive look at cloning research from the University of Utah's Genetic Science Learning Center
- Click and Clone. Try it yourself in the virtual mouse cloning laboratory, from the University of Utah's Genetic Science Learning Center
- "Cloning Addendum: A statement on the cloning report issues by the President's Council on Bioethics" Archived 8 January 2009 at the Wayback Machine. National Review, 15 July 2002 8:45 am
https://en.wikipedia.org/wiki/Cloning
Cloning for warfare
The use of cloning for military purposes has also been explored in several fictional works. In Doctor Who, an alien race of armour-clad, warlike beings called Sontarans was introduced in the 1973 serial "The Time Warrior". Sontarans are depicted as squat, bald creatures who have been genetically engineered for combat. Their weak spot is a "probic vent", a small socket at the back of their neck which is associated with the cloning process.[170] The concept of cloned soldiers being bred for combat was revisited in "The Doctor's Daughter" (2008), when the Doctor's DNA is used to create a female warrior called Jenny.[171]
The 1977 film Star Wars was set against the backdrop of a historical conflict called the Clone Wars. The events of this war were not fully explored until the prequel films Attack of the Clones (2002) and Revenge of the Sith (2005), which depict a space war waged by a massive army of heavily armoured clone troopers that leads to the foundation of the Galactic Empire. Cloned soldiers are "manufactured" on an industrial scale, genetically conditioned for obedience and combat effectiveness. It is also revealed that the popular character Boba Fett originated as a clone of Jango Fett, a mercenary who served as the genetic template for the clone troopers.[172][173]
https://en.wikipedia.org/wiki/Cloning
Cloning as resurrection
Cloning has been used in fiction as a way of recreating historical figures. In the 1976 Ira Levin novel The Boys from Brazil and its 1978 film adaptation, Josef Mengele uses cloning to create copies of Adolf Hitler.[168]
In Michael Crichton's 1990 novel Jurassic Park, which spawned a series of Jurassic Park feature films, the bioengineering company InGen develops a technique to resurrect extinct species of dinosaurs by creating cloned creatures using DNA extracted from fossils. The cloned dinosaurs are used to populate the Jurassic Park wildlife park for the entertainment of visitors. The scheme goes disastrously wrong when the dinosaurs escape their enclosures. Despite being selectively cloned as females to prevent them from breeding, the dinosaurs develop the ability to reproduce through parthenogenesis.[169]
https://en.wikipedia.org/wiki/Cloning
Conservation cloning
Several tissue banks have come into existence, including the "Frozen zoo" at the San Diego Zoo, to store frozen tissue from the world's rarest and most endangered species.[108][114][115][116] This is also referred to as "Conservation cloning".[117][118]
Engineers have proposed a "lunar ark" in 2021 – storing millions of seed, spore, sperm and egg samples from Earth's contemporary species in a network of lava tubes on the Moon as a genetic backup.[119][120][121] Similar proposals have been made since at least 2008.[122] These also include sending human customer DNA,[123] and a proposal for "a lunar backup record of humanity" that includes genetic information by Avi Loeb et al.[124]
Scientists at the University of Newcastle and University of New South Wales announced in March 2013 that the very recently extinct gastric-brooding frog would be the subject of a cloning attempt to resurrect the species.[125]
Many such "De-extinction" projects are being championed by the non-profit Revive & Restore.[126]
https://en.wikipedia.org/wiki/Cloning
Two commonly discussed types of theoretical human cloning are therapeutic cloning and reproductive cloning. Therapeutic cloning would involve cloning cells from a human for use in medicine and transplants, and is an active area of research, but is not in medical practice anywhere in the world, as of 2021. Two common methods of therapeutic cloning that are being researched are somatic-cell nuclear transfer and, more recently, pluripotent stem cell induction. Reproductive cloning would involve making an entire cloned human, instead of just specific cells or tissues.[84]
Ethical issues of cloning
There are a variety of ethical positions regarding the possibilities of cloning, especially human cloning. While many of these views are religious in origin, the questions raised by cloning are faced by secular perspectives as well. Perspectives on human cloning are theoretical, as human therapeutic and reproductive cloning are not commercially used; animals are currently cloned in laboratories and in livestock production.
Advocates support development of therapeutic cloning to generate tissues and whole organs to treat patients who otherwise cannot obtain transplants,[85] to avoid the need for immunosuppressive drugs,[84] and to stave off the effects of aging.[86] Advocates for reproductive cloning believe that parents who cannot otherwise procreate should have access to the technology.[87]
Opponents of cloning have concerns that technology is not yet developed enough to be safe[88] and that it could be prone to abuse (leading to the generation of humans from whom organs and tissues would be harvested),[89][90] as well as concerns about how cloned individuals could integrate with families and with society at large.[91][92] Cloning humans could lead to serious violations of human rights.[93]
Religious groups are divided, with some opposing the technology as usurping "God's place" and, to the extent embryos are used, destroying a human life; others support therapeutic cloning's potential life-saving benefits.[94][95] There is at least one religion, Raëlism, in which cloning plays a major role.[96][97][98]
Contemporary work on this topic is concerned with the ethics, adequate regulation and issues of any cloning carried out by humans, not potentially by extraterrestrials (including in the future), and largely also not replication – also described as mind cloning[99][100][101][102] – of potential whole brain emulations.
Cloning of animals is opposed by animal-groups due to the number of cloned animals that suffer from malformations before they die, and while food from cloned animals has been approved as safe by the US FDA,[103][104] its use is opposed by groups concerned about food safety.[105][106]
In practical terms, the inclusion of "licensing requirements for embryo research projects and fertility clinics, restrictions on the commodification of eggs and sperm, and measures to prevent proprietary interests from monopolizing access to stem cell lines" in international cloning regulations has been proposed, albeit e.g. effective oversight mechanisms or cloning requirements have not been described.[107]
https://en.wikipedia.org/wiki/Cloning
- First artificial parthenogenesis in mammals: (2022) Viable mice offspring was born from unfertilized eggs via targeted DNA methylation editing of seven imprinting control regions.[82]
Human cloning
Human cloning is the creation of a genetically identical copy of a human. The term is generally used to refer to artificial human cloning, which is the reproduction of human cells and tissues. It does not refer to the natural conception and delivery of identical twins. The possibility of human cloning has raised controversies. These ethical concerns have prompted several nations to pass legislation regarding human cloning and its legality. As of right now, scientists have no intention of trying to clone people and they believe their results should spark a wider discussion about the laws and regulations the world needs to regulate cloning.[83]
Two commonly discussed types of theoretical human cloning are therapeutic cloning and reproductive cloning. Therapeutic cloning would involve cloning cells from a human for use in medicine and transplants, and is an active area of research, but is not in medical practice anywhere in the world, as of 2021. Two common methods of therapeutic cloning that are being researched are somatic-cell nuclear transfer and, more recently, pluripotent stem cell induction. Reproductive cloning would involve making an entire cloned human, instead of just specific cells or tissues.[84]
https://en.wikipedia.org/wiki/Cloning
Genome editing, or genome engineering, or gene editing, is a type of genetic engineering in which DNA is inserted, deleted, modified or replaced in the genome of a living organism. Unlike early genetic engineering techniques that randomly inserts genetic material into a host genome, genome editing targets the insertions to site-specific locations. The basic mechanism involved in genetic manipulations through programmable nucleases is the recognition of target genomic loci and binding of effector DNA-binding domain (DBD), double-strand breaks (DSBs) in target DNA by the restriction endonucleases (FokI and Cas), and the repair of DSBs through homology-directed recombination (HDR) or non-homologous end joining (NHEJ).[1][2]
https://en.wikipedia.org/wiki/Genome_editing
- Macaque monkey: (2017) First successful cloning of a primate species using nuclear transfer, with the birth of two live clones named Zhong Zhong and Hua Hua. Conducted in China in 2017, and reported in January 2018.[75][76][77][78] In January 2019, scientists in China reported the creation of five identical cloned gene-edited monkeys, using the same cloning technique that was used with Zhong Zhong and Hua Hua and Dolly the sheep, and the gene-editing Crispr-Cas9 technique allegedly used by He Jiankui in creating the first ever gene-modified human babies Lulu and Nana. The monkey clones were made to study several medical diseases.[79][80]
https://en.wikipedia.org/wiki/Cloning
The first mammalian cloning (resulting in Dolly) had a success rate of 29 embryos per 277 fertilized eggs, which produced three lambs at birth, one of which lived. In a bovine experiment involving 70 cloned calves, one-third of the calves died quite young. The first successfully cloned horse, Prometea, took 814 attempts. Notably, although the first clones were frogs, no adult cloned frog has yet been produced from a somatic adult nucleus donor cell.[35]
https://en.wikipedia.org/wiki/Cloning
Commercial animal cloning is the cloning of animals for commercial purposes, currently, including livestock, competition camels and horses, pets, medical uses, endangered and extinct animals,[1] as first demonstrated in 1996 for Dolly the sheep.
Cloning methods
Moving or copying all (or nearly all) genes from one animal to form a second, genetically nearly identical, animal is usually done through one of three methods: the Roslin technique, the Honolulu technique, and Artificial Twinning.[2] The first two of these involve a process known as somatic cell nuclear transfer.[3] In this process, an oocyte is taken from a surrogate mother and put through enucleation, a process that removes the nucleus from inside the oocyte. Somatic cells are then taken from the animal that is being cloned, transferred into the blank oocyte in order to provide genetic material, and fused with the oocyte using an electrical current. The oocyte is then activated and re-inserted into the surrogate mother. The end result is the formation of an animal that is almost genetically identical to the animal the somatic cells were taken from.[3][4] While somatic cell nuclear transfer was previously believed to only work using genetic material from somatic cells that were unfrozen or were frozen with cryoprotectant (to avoid cell damage caused by freezing), successful dog cloning in various breeds has now been shown using somatic cells from unprotected specimens that had been frozen for up to four days.[5] Another method of cloning includes embryo splitting, the process of taking the blastomeres from a very early animal embryo and separating them before they become differentiated in order to create two or more separate organisms. When using embryo splitting, cloning must occur before the birth of the animal, and clones grow up at the same time (in a similar fashion to monozygotic twins).[3]
Livestock cloning
The US Food and Drug Administration has concluded that "Food from cattle, swine, and goat clones is as safe to eat as food from any other cattle, swine, or goat."[1] It has also noted that "The main use of agricultural clones is to produce breeding stock, not food. Clones allow farmers to upgrade the overall quality of their herds by providing more copies of the best animals in the herd. These animals are then used for conventional breeding, and the sexually reproduced offspring become the food producing animals." Regardless, a large (million cloned cattle per year) Tianjin animal cloning center was proposed in 2015 "to be put into use in the first half of 2016",[6], but as of 2022, no opening had been reported. The goals of cloning listed by the FDA include "disease resistance ... suitability to climate ... quality body type .. fertility ... and market preference (leanness, tenderness, color, size of various cuts, etc.")[1] Milk productivity is another desirable trait that cloning is used for, including in the case of cloned "supercows".[7]
Competition horses and camels
Polo horses[8] and camels[9] are being cloned for competition use. Horse cloning is often referred to as "equine cloning". In 2012, Féderation Equestre Internationale (FEI) lifted a ban on horse cloning, allowing them to compete.[10][11][12]
Medical uses
Organs from cloned pigs are beginning to be transplanted into human patients.[13][better source needed] (See Xenotransplantation)
Cancer-sniffing dogs have also been cloned. A review concluded that "qualified elite working dogs can be produced by cloning a working dog that exhibits both an appropriate temperament and good health."[14]
Other working animals with high performance
Cloning of super sniffer dogs for airports was reported in 2011, four years afterwards when the dogs started working.[15] Cloning of a successful rescue dog was reported in 2009[16] and of a police dog in 2019.[17]
Endangered and extinct animals
The only extinct animal to be cloned as of 2022 is a Pyrenean ibex, born on July 30, 2003, in Spain, which died minutes later due to physical defects in the lungs.[18][19]
Endangered animals, to add genetic diversity to inbred species, are also being cloned in certain centers, notably ViaGen, aided by the San Diego Frozen Zoo, and Revive & Restore.[20] This is also referred to as "conservation cloning".[21][22] Two examples are the black-footed ferret and Przewalski’s horse.[20]
In 2022, the world’s first cloned Arctic wolf "Maya" was born in Beijing by Sinogene. Although Arctic wolves are no longer listed by the International Union for Conservation of Nature’s Red List as an endangered species, the technique could be used to help other animals at risk of extinction, such as Mexican gray wolves and red wolves. The team of Sinogene is confident that the technique could be used to restore lost species or boost numbers in endangered animal populations. [23]
In a recent study using sturgeons (species of fish in the Acipenseridae family), scientists have been making improvements to a technique called somatic nuclear cell transfer with the ultimate goal being to save endangered species. Sturgeons are endangered due to the high levels of poaching, increased destruction to habitats, water pollution, and overfishing. This makes this species a perfect candidate for improving this type of cloning method, in hopes of preserving natural populations from becoming endangered. The somatic nuclear cell transfer technique is a well-known cloning method that has been used for years but focuses on species that are thriving rather than those endangered or extinct animals. This technique usually uses a single somatic donor cell with a single manipulation and inserts it into a recipient egg of the species of interest. It has recently been found that the position by which that somatic cell is located inside the recipient is very important in order to successfully clone a species. By making adjustments to the original method of using a single somatic cell and instead use multiple somatic donor cells to insert into the recipient egg, the likeliness of the somatic donor cells being in the crucial position on the egg will increase tremendously. This increase will then result in higher success rates with cloning. There is still ongoing research using this improved method happening but from the data collected thus far, it seems to be a reasonable method to continue and soon be able to help stop species like the sturgeons from becoming endangered and possibly stop extinction from occurring.[24]
When it comes to the cloning of extinct animals, things become quite difficult because when death occurs, the animal's DNA starts to decay. This makes it impossible to fully preserve the entire genome by known cloning methods. New studies using genome editing, have made it possible to bring back traits of the extinct species. Currently, a lab at Harvard University's Wyss Institute, is working on a way to bring back a famously known extinct species referred to as the mammoth (or at least incorporate its traits into a closely related species). The goal is to genetically modify the mammoth's closely related living species, the elephant, in hopes of expanding available habitats of elephants and connecting lost ecological interactions. In order to make this goal possible, the team must first sequence and assemble the mammoth genome from preserved remains. After identifying the genome sequencing, a comparison of the extinct species and its closest living relative's genome can be made using advanced genome editing technology.
Studies have shown "cloning" of extinct animals is impossible, unlike currently living animals. As a closely related alternative, genome engineering seems to be the next best thing in making these long-gone species somewhat present in today's time. Methods like CRISPR/caps9 allow scientists to edit the genome of closely related relatives, allowing at least the traits of extinct animals to become present, which is essentially like bringing the extinct species back itself.[25]
History and commercialization
ViaGen began by offering cloning to the livestock and equine industry in 2003,[26] and later as ViaGen Pets included cloning of cats and dogs in 2016.[27] ViaGen's subsidiary, stART Licensing, owns a cloning patent which is licensed to their only competitor as of 2018, who also offers animal cloning services.[28] (Viagen is a subsidiary of Precigen.[29])
The first commercially cloned pet was a cat named Little Nicky, produced in 2004 by Genetic Savings & Clone for a north Texas woman for the fee of US$50,000.[30] On May 21, 2008, BioArts International[31] announced a limited commercial dog cloning service (through a program it called Best Friends Again) in partnership with a Korean company named Sooam Biotech. This program came after the announcement of the successful cloning of a family dog named Missy, an achievement widely publicized in the Missyplicity Project. In September 2009, BioArts announced the end of its dog cloning service.[32] In July 2008, the Seoul National University (co-parents of Snuppy, reputedly the world's first cloned dog in 2005) created five clones of a dog named Booger for its Californian owner. The woman paid $50,000 for this service.[33]
Sooam Biotech continued developing proprietary techniques for cloning dogs[34] based on a licence from ViaGen's subsidiary, stART Licensing (which owned the original patent for the process of animal cloning.[35]). (Although the animal itself is not patentable, the process is protected by a patent).[36] Sooam created cloned puppies for owners whose dogs had died, charging $100,000 per clone.[37][38] Sooam Biotech was reported to have cloned approximately 700 dogs by 2015[37] and to be producing 500 cloned embryos of various breeds a day in 2016.[39] In 2015, the longest period after which Sooam Biotech could clone a puppy was 12 days from the death of the original pet dog.[40] Sinogene Biotechnology created the first Chinese clone dog in 2017 before commercializing the cloning service and joining in the pet cloning market.[41] In 2019, Sinogene successfully created the first Chinese cloned cat.[42] In June 2022, "Zhuang Zhuang" was cloned by the Beijing laboratory Sinogene. He is the first from the "warmblood" group of breeds to be born in China and to be officially approved by the China Horse Industry Association.[43]
Controversies
Animal welfare
The mortality rate for cloned animals is higher than for those born of natural processes. This includes a discrepancy pre-birth, during birth, and after birth in survival rates and quality of life, leading to ethical concerns.[44] Many of these discrepancies are thought to come from maternal mRNA already present in the oocyte prior to the transfer of genetic material as well as from DNA methylation, both of which contribute to the development of the animal in the womb of the surrogate.[45] Some common issues seen with cloned animals are shortened telomeres, the repetitive end sequences of DNA whose decreasing length over the lifespan of an organism have been associated with aging;[46] large offspring syndrome, the abnormal size of cloned individuals due to epigenetic (gene expression) changes; and methylation patterns of genetic material that are so abnormal compared to standard embryos of the species being cloned as to be incompatible with life.[45]
Pet cloning
While pet cloning is sometimes advertised as a prospective method for re-gaining a deceased companionship animal, pet cloning does not result in animals that are exactly like the previous pet (in looks or personality).[47] Although the animal in question is cloned, there are still phenotypical differences that may affect its appearance or health. This issue was brought to light in the cloning of a cat named Rainbow. Rainbow's clone, later named CC, was genetically identical to Rainbow, yet CC's coloring patterns were not the same due to the development of the kitten inside the womb as well as random genetic disparities in the clone such as variable X-chromosome inactivation.[48]
Despite its controversies, the study of pet cloning holds the potential to contribute to scientific, veterinary, and medical knowledge, and it is a potential resource in efforts to preserve endangered cousins of the cat and dog.[46]
In 2005, California Assembly Member Lloyd Levine introduced a bill to ban the sale or transfer of pet clones in California.[49] That bill was voted down.[50]
See also
- Biobank
- Cultivar: term used in botany to refer to specific breeds (made using selective cross breeding and sometimes genetic modification) that have distinct properties. Often reproduced using cloning to avoid properties being lost due to sexual propagation.
- List of animals that have been cloned
- Working animal
References
- "Cloned pets escape retail sales ban in California". dvm360 magazine. dvm360. July 1, 2005. Retrieved April 12, 2018.
https://en.wikipedia.org/wiki/Commercial_animal_cloning
Induced pluripotent stem cells (also known as iPS cells or iPSCs) are a type of pluripotent stem cell that can be generated directly from a somatic cell. The iPSC technology was pioneered by Shinya Yamanaka and Kazutoshi Takahashi in Kyoto, Japan, who together showed in 2006 that the introduction of four specific genes (named Myc, Oct3/4, Sox2 and Klf4), collectively known as Yamanaka factors, encoding transcription factors could convert somatic cells into pluripotent stem cells.[1] Shinya Yamanaka was awarded the 2012 Nobel Prize along with Sir John Gurdon "for the discovery that mature cells can be reprogrammed to become pluripotent."[2]
Pluripotent stem cells hold promise in the field of regenerative medicine.[3] Because they can propagate indefinitely, as well as give rise to every other cell type in the body (such as neurons, heart, pancreatic, and liver cells), they represent a single source of cells that could be used to replace those lost to damage or disease.
The most well-known type of pluripotent stem cell is the embryonic stem cell. However, since the generation of embryonic stem cells involves destruction (or at least manipulation)[4] of the pre-implantation stage embryo, there has been much controversy surrounding their use. Patient-matched embryonic stem cell lines can now be derived using somatic cell nuclear transfer (SCNT).
Since iPSCs can be derived directly from adult tissues, they not only bypass the need for embryos, but can be made in a patient-matched manner, which means that each individual could have their own pluripotent stem cell line. These unlimited supplies of autologous cells could be used to generate transplants without the risk of immune rejection. While the iPSC technology has not yet advanced to a stage where therapeutic transplants have been deemed safe, iPSCs are readily being used in personalized drug discovery efforts and understanding the patient-specific basis of disease.[5]
Yamanaka named iPSCs with a lower case "i" due to the popularity of the iPod and other products.[6][7][8][9][10][dubious ]
In his Nobel seminar, Yamanaka cited the earlier seminal work of Harold Weintraub on the role of myoblast determination protein 1 (MyoD) in reprogramming cell fate to a muscle lineage as an important precursor to the discovery of iPSCs.[11]
Production
iPSCs are typically derived by introducing products of specific sets of pluripotency-associated genes, or "reprogramming factors", into a given cell type. The original set of reprogramming factors (also dubbed Yamanaka factors) are the transcription factors Oct4 (Pou5f1), Sox2, Klf4 and cMyc. While this combination is most conventional in producing iPSCs, each of the factors can be functionally replaced by related transcription factors, miRNAs, small molecules, or even non-related genes such as lineage specifiers.[12]It is also clear that pro-mitotic factors such as C-MYC/L-MYC or repression of cell cycle checkpoints, such as p53, are conduits to creating a compliant cellular state for iPSC reprograming .[13]
iPSC derivation is typically a slow and inefficient process, taking 1–2 weeks for mouse cells and 3–4 weeks for human cells, with efficiencies around 0.01–0.1%. However, considerable advances have been made in improving the efficiency and the time it takes to obtain iPSCs. Upon introduction of reprogramming factors, cells begin to form colonies that resemble pluripotent stem cells, which can be isolated based on their morphology, conditions that select for their growth, or through expression of surface markers or reporter genes.
First generation (mouse)
Induced pluripotent stem cells were first generated by Shinya Yamanaka and Kazutoshi Takahashi at Kyoto University, Japan, in 2006.[1] They hypothesized that genes important to embryonic stem cell (ESC) function might be able to induce an embryonic state in adult cells. They chose twenty-four genes previously identified as important in ESCs and used retroviruses to deliver these genes to mouse fibroblasts. The fibroblasts were engineered so that any cells reactivating the ESC-specific gene, Fbx15, could be isolated using antibiotic selection.
Upon delivery of all twenty-four factors, ESC-like colonies emerged that reactivated the Fbx15 reporter and could propagate indefinitely. To identify the genes necessary for reprogramming, the researchers removed one factor at a time from the pool of twenty-four. By this process, they identified four factors, Oct4, Sox2, cMyc, and Klf4, which were each necessary and together sufficient to generate ESC-like colonies under selection for reactivation of Fbx15.
Second generation (mouse)
In June 2007, three separate research groups, including that of Yamanaka's, a Harvard/University of California, Los Angeles collaboration, and a group at MIT, published studies that substantially improved on the reprogramming approach, giving rise to iPSCs that were indistinguishable from ESCs. Unlike the first generation of iPSCs, these second generation iPSCs produced viable chimeric mice and contributed to the mouse germline, thereby achieving the 'gold standard' for pluripotent stem cells.
These second-generation iPSCs were derived from mouse fibroblasts by retroviral-mediated expression of the same four transcription factors (Oct4, Sox2, cMyc, Klf4). However, instead of using Fbx15 to select for pluripotent cells, the researchers used Nanog, a gene that is functionally important in ESCs. By using this different strategy, the researchers created iPSCs that were functionally identical to ESCs.[14][15][16][17]
Human induced pluripotent stem cells
Generation from human fibroblasts
Reprogramming of human cells to iPSCs was reported in November 2007 by two independent research groups: Shinya Yamanaka of Kyoto University, Japan, who pioneered the original iPSC method, and James Thomson of University of Wisconsin-Madison who was the first to derive human embryonic stem cells. With the same principle used in mouse reprogramming, Yamanaka's group successfully transformed human fibroblasts into iPSCs with the same four pivotal genes, Oct4, Sox2, Klf4, and cMyc, using a retroviral system,[18] while Thomson and colleagues used a different set of factors, Oct4, Sox2, Nanog, and Lin28, using a lentiviral system.[19]
Generation from additional cell types
Obtaining fibroblasts to produce iPSCs involves a skin biopsy, and there has been a push towards identifying cell types that are more easily accessible.[20][21] In 2008, iPSCs were derived from human keratinocytes, which could be obtained from a single hair pluck.[22][23] In 2010, iPSCs were derived from peripheral blood cells,[24][25] and in 2012, iPSCs were made from renal epithelial cells in the urine.[26]
Other considerations for starting cell type include mutational load (for example, skin cells may harbor more mutations due to UV exposure),[20][21] time it takes to expand the population of starting cells,[20] and the ability to differentiate into a given cell type.[27]
Genes used to produce iPSCs
The generation of induced pluripotent cells is crucially dependent on the transcription factors used for the induction.
Oct-3/4 and certain products of the Sox gene family (Sox1, Sox2, Sox3, and Sox15) have been identified as crucial transcriptional regulators involved in the induction process whose absence makes induction impossible. Additional genes, however, including certain members of the Klf family (Klf1, Klf2, Klf4, and Klf5), the Myc family (c-myc, L-myc, and N-myc), Nanog, and LIN28, have been identified to increase the induction efficiency.
- Oct-3/4 (Pou5f1) Oct-3/4 is one of the family of octamer ("Oct") transcription factors, and plays a crucial role in maintaining pluripotency. The absence of Oct-3/4 in Oct-3/4+ cells, such as blastomeres and embryonic stem cells, leads to spontaneous trophoblast differentiation, and presence of Oct-3/4 thus gives rise to the pluripotency and differentiation potential of embryonic stem cells. Various other genes in the "Oct" family, including Oct-3/4's close relatives, Oct1 and Oct6, fail to elicit induction, thus demonstrating the exclusiveness of Oct-3/4 to the induction process. However a team headed by Hans Schöler (who discovered the Oct4 gene back in 1989) showed that Oct4 overexpression during reprogramming causes epigenetic changes deteriorating the quality of iPSCs. Comparing to OSKM (Oct4, Sox2, Klf4 and c-Myc) new SKM (Sox2, Klf4 and c-Myc) reprogramming generates iPSCs with developmental potential equivalent to embryonic stem cell, as determined by their ability to generate all-iPSC mice through tetraploid embryo complementation.[28][29]
- Sox family: The Sox family of transcription factors is associated with maintaining pluripotency similar to Oct-3/4, although it is associated with multipotent and unipotent stem cells in contrast with Oct-3/4, which is exclusively expressed in pluripotent stem cells. While Sox2 was the initial gene used for induction by Yamanaka et al., Jaenisch et al., and Thomson et al., other transcription factors in the Sox family have been found to work as well in the induction process. Sox1 yields iPSCs with a similar efficiency as Sox2, and genes Sox3, Sox15, and Sox18 also generate iPSCs, although with decreased efficiency. Velychko et al. engineered a chimeric reprogramming factor, Sox2-17, that enhanced generation of mouse, human, cynomolgus monkey, porcine and bovine iPSCs.[30]
- Klf family: Klf4 of the Klf family of transcription factors was initially identified by Yamanaka et al. and confirmed by Jaenisch et al. As a factor for the generation of mouse iPS cells and was demonstrated by Yamanaka et al. as a factor for generation of human iPS cells. However, Thomson et al. reported that Klf4 was unnecessary for generation of human iPS cells and in fact failed to generate human iPS cells. Klf2 and Klf4 were found to be factors capable of generating iPS cells, and related genes Klf1 and Klf5 did as well, although with reduced efficiency.
- Myc family: The Myc family of transcription factors are proto-oncogenes implicated in cancer. Yamanaka et al. and Jaenisch et al. demonstrated that c-myc is a factor implicated in the generation of mouse iPS cells and Yamanaka et al. demonstrated it was a factor implicated in the generation of human iPS cells. However, Thomson et al., Yamanaka et al. usage of the "myc" family of genes in induction of iPS cells is troubling for the eventuality of iPS cells as clinical therapies, as 25% of mice transplanted with c-myc-induced iPS cells developed lethal teratomas. N-myc and L-myc have been identified to induce instead of c-myc with similar efficiency.
- Nanog: In embryonic stem cells, Nanog, along with Oct-3/4 and Sox2, is necessary in promoting pluripotency. Therefore, it was surprising when Yamanaka et al. reported that Nanog was unnecessary for induction although Thomson et al. has reported it is possible to generate iPS cells with Nanog as one of the factors.
- LIN28: LIN28 is an mRNA binding protein[31] expressed in embryonic stem cells and embryonic carcinoma cells associated with differentiation and proliferation. Thomson et al. demonstrated that LIN28 is a factor in iPSC generation in combination with OCT4, SOX2, and NANOG.[19]
- Glis1: Glis1 is transcription factor that can be used with Oct-3/4, Sox2 and Klf4 to induce pluripotency. It poses numerous advantages when used instead of C-myc.[32]
Challenges in reprogramming cells to pluripotency
Although the methods pioneered by Yamanaka and others have demonstrated that adult cells can be reprogrammed to iPS cells, there are still challenges associated with this technology:
- Low efficiency: in general, the conversion to iPS cells has been incredibly low. For example, the rate at which somatic cells were reprogrammed into iPS cells in Yamanaka's original mouse study was 0.01–0.1%.[1] The low efficiency rate may reflect the need for precise timing, balance, and absolute levels of expression of the reprogramming genes. It may also suggest a need for rare genetic or epigenetic changes in the original somatic cell population or in the prolonged culture. However, recently a path was found for efficient reprogramming which required downregulation of the nucleosome remodeling and deacetylation (NuRD) complex. Overexpression of Mbd3, a subunit of NuRD, inhibits induction of iPSCs. Depletion of Mbd3, on the other hand, improves reprogramming efficiency,[33] that results in deterministic and synchronized iPS cell reprogramming (near 100% efficiency within seven days from mouse and human cells).[34]
- Genomic Insertion: genomic integration of the transcription factors limits the utility of the transcription factor approach because of the risk of mutations being inserted into the target cell's genome.[35] A common strategy for avoiding genomic insertion has been to use a different vector for input. Plasmids, adenoviruses, and transposon vectors have all been explored, but these often come with the tradeoff of lower throughput.[36][37][38]
- Tumorigenicity: Depending on the methods used, reprogramming of adult cells to obtain iPSCs may pose significant risks that could limit their use in humans. For example, if viruses are used to genomically alter the cells, the expression of oncogenes (cancer-causing genes) may potentially be triggered. In February 2008, scientists announced the discovery of a technique that could remove oncogenes after the induction of pluripotency, thereby increasing the potential use of iPS cells in human diseases.[39] In another study, Yamanaka reported that one can create iPSCs without the oncogene c-Myc. The process took longer and was not as efficient, but the resulting chimeras did not develop cancer.[40] Inactivation or deletion of the tumor suppressor p53, which is a key regulator of cancer, significantly increases reprogramming efficiency.[41] Thus there seems to be a tradeoff between reprogramming efficiency and tumor generation.
- Incomplete reprogramming: reprogramming also faces the challenge of completeness. This is particularly challenging because the genome-wide epigenetic code must be reformatted to that of the target cell type in order to fully reprogram a cell. However, three separate groups were able to find mouse embryonic fibroblast (MEF)-derived iPS cells that could be injected into tetraploid blastocysts and resulted in the live birth of mice derived entirely from iPS cells, thus ending the debate over the equivalence of embryonic stem cells (ESCs) and iPS with regard to pluripotency.[42]
The table on the right summarizes the key strategies and techniques used to develop iPS cells in the first five years after Yamanaka et al.'s 2006 breakthrough. Rows of similar colors represent studies that used similar strategies for reprogramming.
Alternative approaches
Mimicking transcription factors with chemicals
One of the main strategies for avoiding problems (1) and (2) has been to use small molecules that can mimic the effects of transcription factors. These compounds can compensate for a reprogramming factor that does not effectively target the genome or fails at reprogramming for another reason; thus they raise reprogramming efficiency. They also avoid the problem of genomic integration, which in some cases contributes to tumor genesis. Key studies using such strategy were conducted in 2008. Melton et al. studied the effects of histone deacetylase (HDAC) inhibitor valproic acid. They found that it increased reprogramming efficiency 100-fold (compared to Yamanaka's traditional transcription factor method).[43] The researchers proposed that this compound was mimicking the signaling that is usually caused by the transcription factor c-Myc. A similar type of compensation mechanism was proposed to mimic the effects of Sox2. In 2008, Ding et al. used the inhibition of histone methyl transferase (HMT) with BIX-01294 in combination with the activation of calcium channels in the plasma membrane in order to increase reprogramming efficiency.[44] Deng et al. of Beijing University reported in July 2013 that induced pluripotent stem cells can be created without any genetic modification. They used a cocktail of seven small-molecule compounds including DZNep to induce the mouse somatic cells into stem cells which they called CiPS cells with the efficiency – at 0.2% – comparable to those using standard iPSC production techniques. The CiPS cells were introduced into developing mouse embryos and were found to contribute to all major cells types, proving its pluripotency.[45][46]
Ding et al. demonstrated an alternative to transcription factor reprogramming through the use of drug-like chemicals. By studying the MET (mesenchymal-epithelial transition) process in which fibroblasts are pushed to a stem-cell like state, Ding's group identified two chemicals – ALK5 inhibitor SB431412 and MEK (mitogen-activated protein kinase) inhibitor PD0325901 – which was found to increase the efficiency of the classical genetic method by 100 fold. Adding a third compound known to be involved in the cell survival pathway, Thiazovivin further increases the efficiency by 200 fold. Using the combination of these three compounds also decreased the reprogramming process of the human fibroblasts from four weeks to two weeks.[47][48]
In April 2009, it was demonstrated that generation of iPS cells is possible without any genetic alteration of the adult cell: a repeated treatment of the cells with certain proteins channeled into the cells via poly-arginine anchors was sufficient to induce pluripotency.[49] The acronym given for those iPSCs is piPSCs (protein-induced pluripotent stem cells).
Alternate vectors
Another key strategy for avoiding problems such as tumorgenesis and low throughput has been to use alternate forms of vectors: adenovirus, plasmids, and naked DNA or protein compounds.
In 2008, Hochedlinger et al. used an adenovirus to transport the requisite four transcription factors into the DNA of skin and liver cells of mice, resulting in cells identical to ESCs. The adenovirus is unique from other vectors like viruses and retroviruses because it does not incorporate any of its own genes into the targeted host and avoids the potential for insertional mutagenesis.[44] In 2009, Freed et al. demonstrated successful reprogramming of human fibroblasts to iPS cells.[50] Another advantage of using adenoviruses is that they only need to present for a brief amount of time in order for effective reprogramming to take place.
Also in 2008, Yamanaka et al. found that they could transfer the four necessary genes with a plasmid.[36] The Yamanaka group successfully reprogrammed mouse cells by transfection with two plasmid constructs carrying the reprogramming factors; the first plasmid expressed c-Myc, while the second expressed the other three factors (Oct4, Klf4, and Sox2). Although the plasmid methods avoid viruses, they still require cancer-promoting genes to accomplish reprogramming. The other main issue with these methods is that they tend to be much less efficient compared to retroviral methods. Furthermore, transfected plasmids have been shown to integrate into the host genome and therefore they still pose the risk of insertional mutagenesis. Because non-retroviral approaches have demonstrated such low efficiency levels, researchers have attempted to effectively rescue the technique with what is known as the PiggyBac Transposon System. Several studies have demonstrated that this system can effectively deliver the key reprogramming factors without leaving footprint mutations in the host cell genome. The PiggyBac Transposon System involves the re-excision of exogenous genes, which eliminates the issue of insertional mutagenesis.[citation needed]
Stimulus-triggered acquisition of pluripotency cell
In January 2014, two articles were published claiming that a type of pluripotent stem cell can be generated by subjecting the cells to certain types of stress (bacterial toxin, a low pH of 5.7, or physical squeezing); the resulting cells were called STAP cells, for stimulus-triggered acquisition of pluripotency.[51]
In light of difficulties that other labs had replicating the results of the surprising study, in March 2014, one of the co-authors has called for the articles to be retracted.[52] On 4 June 2014, the lead author, Obokata agreed to retract both the papers [53] after she was found to have committed 'research misconduct' as concluded in an investigation by RIKEN on 1 April 2014.[54]
RNA molecules
MicroRNAs are short RNA molecules that bind to complementary sequences on messenger RNA and block expression of a gene. Measuring variations in microRNA expression in iPS cells can be used to predict their differentiation potential.[55] Addition of microRNAs can also be used to enhance iPS potential. Several mechanisms have been proposed.[55] ES cell-specific microRNA molecules (such as miR-291, miR-294 and miR-295) enhance the efficiency of induced pluripotency by acting downstream of c-Myc.[56] microRNAs can also block expression of repressors of Yamanaka's four transcription factors, and there may be additional mechanisms induce reprogramming even in the absence of added exogenous transcription factors.[55]
Identity
Induced pluripotent stem cells are similar to natural pluripotent stem cells, such as embryonic stem cells, in many aspects, such as the expression of certain stem cell genes and proteins, chromatin methylation patterns, doubling time, embryoid body formation, teratoma formation, viable chimera formation, and potency and differentiability, but the full extent of their relation to natural pluripotent stem cells is still being assessed.[1]
Gene expression and genome-wide H3K4me3 and H3K27me3 were found to be extremely similar between ES and iPS cells.[57][citation needed] The generated iPSCs were remarkably similar to naturally isolated pluripotent stem cells (such as mouse and human embryonic stem cells, mESCs and hESCs, respectively) in the following respects, thus confirming the identity, authenticity, and pluripotency of iPSCs to naturally isolated pluripotent stem cells:
- Cellular biological properties
- Morphology: iPSCs were morphologically similar to ESCs. Each cell had round shape, large nucleolus and scant cytoplasm. Colonies of iPSCs were also similar to that of ESCs. Human iPSCs formed sharp-edged, flat, tightly packed colonies similar to hESCs and mouse iPSCs formed the colonies similar to mESCs, less flat and more aggregated colonies than that of hESCs.
- Growth properties: Doubling time and mitotic activity are cornerstones of ESCs, as stem cells must self-renew as part of their definition. iPSCs were mitotically active, actively self-renewing, proliferating, and dividing at a rate equal to ESCs.
- Stem cell markers: iPSCs expressed cell surface antigenic markers expressed on ESCs. Human iPSCs expressed the markers specific to hESC, including SSEA-3, SSEA-4, TRA-1-60, TRA-1-81, TRA-2-49/6E, and Nanog. Mouse iPSCs expressed SSEA-1 but not SSEA-3 nor SSEA-4, similarly to mESCs.
- Stem Cell Genes: iPSCs expressed genes expressed in undifferentiated ESCs, including Oct-3/4, Sox2, Nanog, GDF3, REX1, FGF4, ESG1, DPPA2, DPPA4, and hTERT.
- Telomerase activity: Telomerases are necessary to sustain cell division unrestricted by the Hayflick limit of ~50 cell divisions. hESCs express high telomerase activity to sustain self-renewal and proliferation, and iPSCs also demonstrate high telomerase activity and express hTERT (human telomerase reverse transcriptase), a necessary component in the telomerase protein complex.
- Pluripotency: iPSCs were capable of differentiation in a fashion similar to ESCs into fully differentiated tissues.
- Neural differentiation: iPSCs were differentiated into neurons, expressing βIII-tubulin, tyrosine hydroxylase, AADC, DAT, ChAT, LMX1B, and MAP2. The presence of catecholamine-associated enzymes may indicate that iPSCs, like hESCs, may be differentiable into dopaminergic neurons. Stem cell-associated genes were downregulated after differentiation.
- Cardiac differentiation: iPSCs were differentiated into cardiomyocytes that spontaneously began beating. Cardiomyocytes expressed TnTc, MEF2C, MYL2A, MYHCβ, and NKX2.5. Stem cell-associated genes were downregulated after differentiation.
- Teratoma formation: iPSCs injected into immunodeficient mice spontaneously formed teratomas after nine weeks. Teratomas are tumors of multiple lineages containing tissue derived from the three germ layers endoderm, mesoderm and ectoderm; this is unlike other tumors, which typically are of only one cell type. Teratoma formation is a landmark test for pluripotency.
- Embryoid body: hESCs in culture spontaneously form ball-like embryo-like structures termed "embryoid bodies", which consist of a core of mitotically active and differentiating hESCs and a periphery of fully differentiated cells from all three germ layers. iPSCs also form embryoid bodies and have peripheral differentiated cells.
- Chimeric mice: hESCs naturally reside within the inner cell mass (embryoblast) of blastocysts, and in the embryoblast, differentiate into the embryo while the blastocyst's shell (trophoblast) differentiates into extraembryonic tissues. The hollow trophoblast is unable to form a living embryo, and thus it is necessary for the embryonic stem cells within the embryoblast to differentiate and form the embryo. iPSCs were injected by micropipette into a trophoblast, and the blastocyst was transferred to recipient females. Chimeric living mouse pups were created: mice with iPSC derivatives incorporated all across their bodies with 10–90% chimerism.
- Tetraploid complementation: iPS cells from mouse fetal fibroblasts injected into tetraploid blastocysts (which themselves can only form extra-embryonic tissues) can form whole, non-chimeric, fertile mice, although with low success rate.[42][58][59]
- Epigenetic reprogramming
- Promoter demethylation: Methylation is the transfer of a methyl group to a DNA base, typically the transfer of a methyl group to a cytosine molecule in a CpG site (adjacent cytosine/guanine sequence). Widespread methylation of a gene interferes with expression by preventing the activity of expression proteins, or by recruiting enzymes that interfere with expression. Thus, methylation of a gene effectively silences it by preventing transcription. Promoters of pluripotency-associated genes, including Oct-3/4, Rex1, and Nanog, were demethylated in iPSCs, demonstrating their promoter activity and the active promotion and expression of pluripotency-associated genes in iPSCs.
- DNA methylation globally: Human iPS cells are highly similar to ES cells in their patterns of which cytosines are methylated, more than to any other cell type. However, on the order of a thousand sites show differences in several iPS cell lines. Half of these resemble the somatic cell line the iPS cells were derived from, the rest are iPSC-specific. Tens of regions which are megabases in size have also been found where iPS cells are not reprogrammed to the ES cell state.[60]
- Histone demethylation: Histones are compacting proteins that are structurally localized to DNA sequences that can affect their activity through various chromatin-related modifications. H3 histones associated with Oct-3/4, Sox2, and Nanog were demethylated, indicating the expression of Oct-3/4, Sox2, and Nanog.
Safety
- The major concern with the potential clinical application of iPSCs is their propensity to form tumors.[61] Much the same as ESC, iPSCs readily form teratoma when injected into immunodeficient mice. Teratoma formation is considered a major obstacle to stem-cell based regenerative medicine by the FDA.
- A more recent study on motor functional recovery after spinal cord injuries in mice showed that after human-induced pluripotent stem cells were transplanted into the mice, the cells differentiated into three neural lineages in the spinal cord. The cells stimulated regrowth of the damaged spinal cord, maintained myelination, and formed synapses. These positive outcomes were observed for over 112 days after the spinal cord injury, without tumor formation.[62] Nevertheless, a follow-up study by the same group showed distinct clones of human-induced pluripotent stem cells eventually formed tumors.[63]
- Since iPSCs can only be produced with high efficiency at this time using modifications, they are generally predicted to be less safe and more tumorigenic than hESC. All the genes that have been shown to promote iPSC formation have also been linked to cancer in one way or another. Some of the genes are known oncogenes, including the members of the Myc family. While omitting Myc still allows for IPSC formation, the efficiency is reduced up to 100 fold.
- A non-genetic method of producing iPSCs has been demonstrated using recombinant proteins, but its efficiency was quite low.[49] However, refinements to this methodology yielding higher efficiency may lead to production of safer iPSCs. Other approaches such as using adenovirus or plasmids are generally thought to be safer than retroviral methods.
- An important area for future studies in the iPSC field is directly testing iPSC tumorigenicity using methods that mimic the approaches that would be used for regenerative medicine therapies. Such studies are crucial since iPSCs not only form teratoma, but also mice derived from iPSCs have a high incidence of death from malignant cancer.[64] A 2010 paper was published in the journal Stem Cells indicating that iPS cells are far more tumorigenic than ESC, supporting the notion that iPS cell safety is a serious concern.[65]
- Concern regarding the immunogenicity of IPS cells arose in 2011 when Zhou et al. performed a study involving a teratoma formation assay and demonstrated that IPS cells produced an immune response strong enough to cause rejection of the cells. When a similar procedure was performed on genetically equivalent ES cells however, Zhou et al. found teratomas, which indicated that the cells were tolerated by the immune system.[66] In 2013, Araki et al. attempted to reproduce the conclusion obtained by Zhou et al. using a different procedure. They took cells from a chimera that had been grown from IPSC clones and a mouse embryo, this tissue was then transplanted into syngenic mice. They conducted a similar trial using ES cells instead of IPSC clone and compared the results. Findings indicate that there was no significant difference in the immunogenic response produced by the IPS cells and the ES cells. Furthermore, Araki et al. reported little or no immunogenic response for both cell lines.[67] Thus, Araki et al. was unable to come to the same conclusion as Zhou et al.
Recent achievements and future tasks for safe iPSC-based cell therapy are collected in the review of Okano et al.[68]
Medical research
The task of producing iPS cells continues to be challenging due to the six problems mentioned above. A key tradeoff to overcome is that between efficiency and genomic integration. Most methods that do not rely on the integration of transgenes are inefficient, while those that do rely on the integration of transgenes face the problems of incomplete reprogramming and tumor genesis, although a vast number of techniques and methods have been attempted. Another large set of strategies is to perform a proteomic characterization of iPS cells.[59] Further studies and new strategies should generate optimal solutions to the five main challenges. One approach might attempt to combine the positive attributes of these strategies into an ultimately effective technique for reprogramming cells to iPS cells.
Another approach is the use of iPS cells derived from patients to identify therapeutic drugs able to rescue a phenotype. For instance, iPS cell lines derived from patients affected by ectodermal dysplasia syndrome (EEC), in which the p63 gene is mutated, display abnormal epithelial commitment that could be partially rescued by a small compound.[69]
Disease modeling and drug development
An attractive feature of human iPS cells is the ability to derive them from adult patients to study the cellular basis of human disease. Since iPS cells are self-renewing and pluripotent, they represent a theoretically unlimited source of patient-derived cells which can be turned into any type of cell in the body. This is particularly important because many other types of human cells derived from patients tend to stop growing after a few passages in laboratory culture. iPS cells have been generated for a wide variety of human genetic diseases, including common disorders such as Down syndrome and polycystic kidney disease.[70][71] In many instances, the patient-derived iPS cells exhibit cellular defects not observed in iPS cells from healthy subjects, providing insight into the pathophysiology of the disease.[72] An international collaborated project, StemBANCC, was formed in 2012 to build a collection of iPS cell lines for drug screening for a variety of disease. Managed by the University of Oxford, the effort pooled funds and resources from 10 pharmaceutical companies and 23 universities. The goal is to generate a library of 1,500 iPS cell lines which will be used in early drug testing by providing a simulated human disease environment.[73] Furthermore, combining hiPSC technology and small molecule or genetically encoded voltage and calcium indicators provided a large-scale and high-throughput platform for cardiovascular drug safety screening.[74][75][76][77]
Organ synthesis
A proof-of-concept of using induced pluripotent stem cells (iPSCs) to generate human organ for transplantation was reported by researchers from Japan. Human 'liver buds' (iPSC-LBs) were grown from a mixture of three different kinds of stem cells: hepatocytes (for liver function) coaxed from iPSCs; endothelial stem cells (to form lining of blood vessels) from umbilical cord blood; and mesenchymal stem cells (to form connective tissue). This new approach allows different cell types to self-organize into a complex organ, mimicking the process in fetal development. After growing in vitro for a few days, the liver buds were transplanted into mice where the 'liver' quickly connected with the host blood vessels and continued to grow. Most importantly, it performed regular liver functions including metabolizing drugs and producing liver-specific proteins. Further studies will monitor the longevity of the transplanted organ in the host body (ability to integrate or avoid rejection) and whether it will transform into tumors.[78][79] Using this method, cells from one mouse could be used to test 1,000 drug compounds to treat liver disease, and reduce animal use by up to 50,000.[80]
Organ regeneration
In 2021, a switchable Yamanaka factors-reprogramming-based approach for regeneration of damaged heart without tumor-formation was demonstrated in mice and was successful if the intervention was carried out immediately before or after a heart attack.[81]
Tissue repair
Embryonic cord-blood cells were induced into pluripotent stem cells using plasmid DNA. Using cell surface endothelial/pericytic markers CD31 and CD146, researchers identified 'vascular progenitor', the high-quality, multipotent vascular stem cells. After the iPS cells were injected directly into the vitreous of the damaged retina of mice, the stem cells engrafted into the retina, grew and repaired the vascular vessels.[82][83]
Labelled iPSCs-derived NSCs injected into laboratory animals with brain lesions were shown to migrate to the lesions and some motor function improvement was observed.[84]
Cardiomyocytes
Beating cardiac muscle cells, iPSC-derived cardiomyocytes, can be mass-produced using chemically defined differentiation protocols.[85][86] These protocols typically modulate the same developmental signaling pathways required for heart development .[87] These iPSC-cardiomyocytes can recapitulate genetic arrhythmias and cardiac drug responses, since they exhibit the same genetic background as the patient from which they were derived.[88][89][90]
In June 2014, Takara Bio received technology transfer from iHeart Japan, a venture company from Kyoto University's iPS Cell Research Institute, to make it possible to exclusively use technologies and patents that induce differentiation of iPS cells into cardiomyocytes in Asia. The company announced the idea of selling cardiomyocytes to pharmaceutical companies and universities to help develop new drugs for heart disease.[91]
On March 9, 2018, the Specified Regenerative Medicine Committee of Osaka University officially approved the world's first clinical research plan to transplant a "myocardial sheet" made from iPS cells into the heart of patients with severe heart failure. Osaka University announced that it had filed an application with the Ministry of Health, Labor and Welfare on the same day.
On May 16, 2018, the clinical research plan was approved by the Ministry of Health, Labor and Welfare's expert group with a condition.[92][93]
In October 2019, a group at Okayama University developed a model of ischemic heart disease using cardiomyocytes differentiated from iPS cells.[94]
Red blood cells
Although a pint of donated blood contains about two trillion red blood cells and over 107 million blood donations are collected globally, there is still a critical need for blood for transfusion. In 2014, type O red blood cells were synthesized at the Scottish National Blood Transfusion Service from iPSC. The cells were induced to become a mesoderm and then blood cells and then red blood cells. The final step was to make them eject their nuclei and mature properly. Type O can be transfused into all patients. Human clinical trials were not expected to begin before 2016.[95]
Clinical trial
The first human clinical trial using autologous iPSCs was approved by the Japan Ministry Health and was to be conducted in 2014 at the Riken Center for Developmental Biology in Kobe. However the trial was suspended after Japan's new regenerative medicine laws came into effect in November 2015.[96] More specifically, an existing set of guidelines was strengthened to have the force of law (previously mere recommendations).[97] iPSCs derived from skin cells from six patients with wet age-related macular degeneration were reprogrammed to differentiate into retinal pigment epithelial (RPE) cells. The cell sheet would be transplanted into the affected retina where the degenerated RPE tissue was excised. Safety and vision restoration monitoring were to last one to three years.[98][99]
In March 2017, a team led by Masayo Takahashi completed the first successful transplant of iPS-derived retinal cells from a donor into the eye of a person with advanced macular degeneration.[100] However it was reported that they are now having complications.[101] The benefits of using autologous iPSCs are that there is theoretically no risk of rejection and that it eliminates the need to use embryonic stem cells. However, these iPSCs were derived from another person.[99]
New clinical trials involving iPSCs are now ongoing not only in Japan, but also in the US and Europe.[102] Research in 2021 on the trial registry Clinicaltrials.gov identified 129 trial listings mentioning iPSCs, but most were non-interventional.[103]
Strategy for obtaining universal iPSCs
To make iPSC-based regenerative medicine technologies available to more patients, it is necessary to create universal iPSCs that can be transplanted independently of haplotypes of HLA. The current strategy for the creation of universal iPSCs has two main goals: to remove HLA expression and to prevent NK cells attacks due to deletion of HLA. Deletion of the B2M and CIITA genes using the CRISPR/Cas9 system has been reported to suppress the expression of HLA class I and class II, respectively. To avoid NK cell attacks. transduction of ligands inhibiting NK-cells, such as HLA-E and CD47 has been used.[104] HLA-C is left unchanged, since the 12 common HLA-C alleles are enough to cover 95% of the world's population.[104]
Anti-aging properties
A multipotent mesenchymal stem cell, when induced into pluripotence, holds great promise to slow or reverse aging phenotypes. Such anti-aging properties were demonstrated in early clinical trials in 2017.[105] In 2020, Stanford University researchers concluded after studying elderly mice that old human cells when subjected to the Yamanaka factors, might rejuvenate and become nearly indistinguishable from their younger counterparts.[106]
See also
- Induced stem cells
- Stem cell treatments
- Stimulus-triggered acquisition of pluripotency cell, a now-discredited claim of pluripotent stem cell generation by immersing cells in an acid
- Induced pluripotent stem cells vs embryonic stem cells lines obtained by SCNT (discussion)
- Dedifferentiation
- Directed differentiation
- Pluripotency
References
- Sarkar TJ, Quarta M, Mukherjee S, Colville A, Paine P, Doan L, et al. (March 2020). "Transient non-integrative expression of nuclear reprogramming factors promotes multifaceted amelioration of aging in human cells". Nature Communications. 11 (1): 1545. Bibcode:2020NatCo..11.1545S. doi:10.1038/s41467-020-15174-3. PMC 7093390. PMID 32210226.
External links
- Center for iPS Cell Research and Application, Kyoto University
- With few factors, adult cells take on character of embryonic stem cells
- Generating iPS Cells from MEFS through Forced Expression of Sox-2, Oct-4, c-Myc, and Klf4
- 2 Minute Video from BSCRF about Induced Pluripotent Stem Cells
- 20Minute Video / The Discovery and Future of Induced Pluripotent Stem (iPS) Cells by Yamanaka January 8, 2008
- Fact sheet on reprogramming
- University of Oxford practical workshop on pluripotent stem cell technology Archived 8 April 2016 at the Wayback Machine
- Allen Cell Explorer - realistic, data-driven 3D visualization of a living hiPSC in its pluri-potent state
- CamBioScience iPSC course Archived 23 April 2019 at the Wayback Machine
https://en.wikipedia.org/wiki/Induced_pluripotent_stem_cell
https://en.wikipedia.org/wiki/Commercial_animal_cloning
In genetics and developmental biology, somatic cell nuclear transfer (SCNT) is a laboratory strategy for creating a viable embryo from a body cell and an egg cell. The technique consists of taking an denucleated oocyte (egg cell) and implanting a donor nucleus from a somatic (body) cell. It is used in both therapeutic and reproductive cloning. In 1996, Dolly the sheep became famous for being the first successful case of the reproductive cloning of a mammal.[1] In January 2018, a team of scientists in Shanghai announced the successful cloning of two female crab-eating macaques (named Zhong Zhong and Hua Hua) from foetal nuclei.[2]
"Therapeutic cloning" refers to the potential use of SCNT in regenerative medicine; this approach has been championed as an answer to the many issues concerning embryonic stem cells (ESCs) and the destruction of viable embryos for medical use, though questions remain on how homologous the two cell types truly are.
Introduction
Somatic cell nuclear transfer is a technique for cloning in which the nucleus of a somatic cell is transferred to the cytoplasm of an enucleated egg. After the somatic cell transfers, the cytoplasmic factors affect the nucleus to become a zygote. The blastocyst stage is developed by the egg to help create embryonic stem cells from the inner cell mass of the blastocyst.[3] The first animal to be developed by this technique was Dolly, the sheep, in 1996.[4]
Process
The process of somatic cell nuclear transfer involves two different cells. The first being a female gamete, known as the ovum (egg/oocyte). In human SCNT experiments, these eggs are obtained through consenting donors, utilizing ovarian stimulation. The second being a somatic cell, referring to the cells of the human body. Skin cells, fat cells, and liver cells are only a few examples. The genetic material of the donor egg cell is removed and discarded, leaving it 'deprogrammed.' What is left is a somatic cell and an enucleated egg cell. These are then fused by inserting the somatic cell into the 'empty' ovum.[5] After being inserted into the egg, the somatic cell nucleus is reprogrammed by its host egg cell. The ovum, now containing the somatic cell's nucleus, is stimulated with a shock and will begin to divide. The egg is now viable and capable of producing an adult organism containing all necessary genetic information from just one parent. Development will ensue normally and after many mitotic divisions, the single cell forms a blastocyst (an early stage embryo with about 100 cells) with an identical genome to the original organism (i.e. a clone).[6] Stem cells can then be obtained by the destruction of this clone embryo for use in therapeutic cloning or in the case of reproductive cloning the clone embryo is implanted into a host mother for further development and brought to term.
Applications
Stem cell research
Somatic cell nuclear transplantation has become a focus of study in stem cell research. The aim of carrying out this procedure is to obtain pluripotent cells from a cloned embryo. These cells genetically matched the donor organism from which they came. This gives them the ability to create patient specific pluripotent cells, which could then be used in therapies or disease research.[7]
Embryonic stem cells are undifferentiated cells of an embryo. These cells are deemed to have a pluripotent potential because they have the ability to give rise to all of the tissues found in an adult organism. This ability allows stem cells to create any cell type, which could then be transplanted to replace damaged or destroyed cells. Controversy surrounds human ESC work due to the destruction of viable human embryos, leading scientists to seek alternative methods of obtaining pluripotent stem cells, SCNT is one such method.
A potential use of stem cells genetically matched to a patient would be to create cell lines that have genes linked to a patient's particular disease. By doing so, an in vitro model could be created, would be useful for studying that particular disease, potentially discovering its pathophysiology, and discovering therapies.[8] For example, if a person with Parkinson's disease donated their somatic cells, the stem cells resulting from SCNT would have genes that contribute to Parkinson's disease. The disease specific stem cell lines could then be studied in order to better understand the condition.[9]
Another application of SCNT stem cell research is using the patient specific stem cell lines to generate tissues or even organs for transplant into the specific patient.[10] The resulting cells would be genetically identical to the somatic cell donor, thus avoiding any complications from immune system rejection.[9][11]
Only a handful of the labs in the world are currently using SCNT techniques in human stem cell research. In the United States, scientists at the Harvard Stem Cell Institute, the University of California San Francisco, the Oregon Health & Science University,[12] Stemagen (La Jolla, CA) and possibly Advanced Cell Technology are currently researching a technique to use somatic cell nuclear transfer to produce embryonic stem cells.[13] In the United Kingdom, the Human Fertilisation and Embryology Authority has granted permission to research groups at the Roslin Institute and the Newcastle Centre for Life.[14] SCNT may also be occurring in China.[15]
In 2005, a South Korean research team led by Professor Hwang Woo-suk, published claims to have derived stem cell lines via SCNT,[16] but supported those claims with fabricated data.[17] Recent evidence has proved that he in fact created a stem cell line from a parthenote.[18][19]
Though there has been numerous successes with cloning animals, questions remain concerning the mechanisms of reprogramming in the ovum. Despite many attempts, success in creating human nuclear transfer embryonic stem cells has been limited. There lies a problem in the human cell's ability to form a blastocyst; the cells fail to progress past the eight cell stage of development. This is thought to be a result from the somatic cell nucleus being unable to turn on embryonic genes crucial for proper development. These earlier experiments used procedures developed in non-primate animals with little success.
A research group from the Oregon Health & Science University demonstrated SCNT procedures developed for primates successfully using skin cells. The key to their success was utilizing oocytes in metaphase II (MII) of the cell cycle. Egg cells in MII contain special factors in the cytoplasm that have a special ability in reprogramming implanted somatic cell nuclei into cells with pluripotent states. When the ovum's nucleus is removed, the cell loses its genetic information. This has been blamed for why enucleated eggs are hampered in their reprogramming ability. It is theorized the critical embryonic genes are physically linked to oocyte chromosomes, enucleation negatively affects these factors. Another possibility is removing the egg nucleus or inserting the somatic nucleus causes damage to the cytoplast, affecting reprogramming ability.
Taking this into account the research group applied their new technique in an attempt to produce human SCNT stem cells. In May 2013, the Oregon group reported the successful derivation of human embryonic stem cell lines derived through SCNT, using fetal and infant donor cells. Using MII oocytes from volunteers and their improved SCNT procedure, human clone embryos were successfully produced. These embryos were of poor quality, lacking a substantial inner cell mass and poorly constructed trophectoderm. The imperfect embryos prevented the acquisition of human ESC. The addition of caffeine during the removal of the ovum's nucleus and fusion of the somatic cell and the egg improved blastocyst formation and ESC isolation. The ESC obtain were found to be capable of producing teratomas, expressed pluripotent transcription factors, and expressed a normal 46XX karyotype, indicating these SCNT were in fact ESC-like.[12] This was the first instance of successfully using SCNT to reprogram human somatic cells. This study used fetal and infantile somatic cells to produce their ESC.
In April 2014, an international research team expanded on this break through. There remained the question of whether the same success could be accomplished using adult somatic cells. Epigenetic and age related changes were thought to possibly hinder an adult somatic cells ability to be reprogrammed. Implementing the procedure pioneered by the Oregon research group they indeed were able to grow stem cells generated by SCNT using adult cells from two donors aged 35 and 75, indicating that age does not impede a cell's ability to be reprogrammed.[20][21]
Late April 2014, the New York Stem Cell Foundation was successful in creating SCNT stem cells derived from adult somatic cells. One of these lines of stem cells was derived from the donor cells of a type 1 diabetic. The group was then able to successfully culture these stem cells and induce differentiation. When injected into mice, cells of all three of the germ layers successfully formed. The most significant of these cells, were those who expressed insulin and were capable of secreting the hormone.[22] These insulin producing cells could be used for replacement therapy in diabetics, demonstrating real SCNT stem cell therapeutic potential.
The impetus for SCNT-based stem cell research has been decreased by the development and improvement of alternative methods of generating stem cells. Methods to reprogram normal body cells into pluripotent stem cells were developed in humans in 2007. The following year, this method achieved a key goal of SCNT-based stem cell research: the derivation of pluripotent stem cell lines that have all genes linked to various diseases.[23] Some scientists working on SCNT-based stem cell research have recently moved to the new methods of induced pluripotent stem cells. Though recent studies have put in question how similar iPS cells are to embryonic stem cells. Epigenetic memory in iPS affects the cell lineage it can differentiate into. For instance, an iPS cell derived from a blood cell using only the yamanaka factors will be more efficient at differentiating into blood cells, while it will be less efficient at creating a neuron.[24] Recent studies indicate however that changes to the epigenetic memory of iPSCs using small molecules can reset them to an almost naive state of pluripotency.[25][26] Studies have even shown that via tetraploid complementation, an entire viable organism can be created solely from iPSCs.[27] SCNT stem cells have been found to have similar challenges. The cause for low yields in bovine SCNT cloning has, in recent years, been attributed to the previously hidden epigenetic memory of the somatic cells that were being introduced into the oocyte.[28]
Reproductive cloning
This technique is currently the basis for cloning animals (such as the famous Dolly the sheep),[29] and has been proposed as a possible way to clone humans. Using SCNT in reproductive cloning has proven difficult with limited success. High fetal and neonatal death make the process very inefficient. Resulting cloned offspring are also plagued with development and imprinting disorders in non-human species. For these reasons, along with moral and ethical objections, reproductive cloning in humans is proscribed in more than 30 countries.[30] Most researchers believe that in the foreseeable future it will not be possible to use the current cloning technique to produce a human clone that will develop to term. It remains a possibility, though critical adjustments will be required to overcome current limitations during early embryonic development in human SCNT.[31][32]
There is also the potential for treating diseases associated with mutations in mitochondrial DNA. Recent studies show SCNT of the nucleus of a body cell afflicted with one of these diseases into a healthy oocyte prevents the inheritance of the mitochondrial disease. This treatment does not involve cloning but would produce a child with three genetic parents. A father providing a sperm cell, one mother providing the egg nucleus, and another mother providing the enucleated egg cell.[10]
In 2018, the first successful cloning of primates using somatic cell nuclear transfer, the same method as Dolly the sheep, with the birth of two live female clones (crab-eating macaques named Zhong Zhong and Hua Hua) was reported.[2][33][34][35][36]
Interspecies nuclear transfer
Interspecies nuclear transfer (iSCNT) is a means of somatic cell nuclear transfer being used to facilitate the rescue of endangered species, or even to restore species after their extinction. The technique is similar to SCNT cloning which typically is between domestic animals and rodents, or where there is a ready supply of oocytes and surrogate animals. However, the cloning of highly endangered or extinct species requires the use of an alternative method of cloning. Interspecies nuclear transfer utilizes a host and a donor of two different organisms that are closely related species and within the same genus. In 2000, Robert Lanza was able to produce a cloned fetus of a gaur, Bos gaurus, combining it successfully with a domestic cow, Bos taurus.[37]
Interspecies nuclear transfer provides evidence of the universality of the triggering mechanism of the cell nucleus reprogramming. For example, Gupta et al.,[38] explored the possibility of producing transgenic cloned embryos by interspecies somatic cell nuclear transfer (iSCNT) of cattle, mice, and chicken donor cells into enucleated pig oocytes. Moreover, NCSU23 medium, which was designed for in vitro culture of pig embryos, was able to support the in vitro development of cattle, mice, and chicken iSCNT embryos up to the blastocyst stage. Furthermore, ovine oocyte cytoplast may be used for remodeling and reprogramming of human somatic cells back to the embryonic stage.[39]
In 2017, the first cloned Bactrian camel was born through iSCNT, using oocytes of dromedary camel and skin fibroblast cells of an adult Bactrian camel as donor nuclei.[40]
Limitations
This section needs additional citations for verification. (August 2016) |
Somatic cell nuclear transfer (SCNT) can be inefficient due to stresses placed on both the egg cell and the introduced nucleus. This can result in a low percentage of successfully reprogrammed cells. For example, in 1996 Dolly the sheep was born after 277 eggs were used for SCNT, which created 29 viable embryos, giving it a measly 0.3% efficiency.[41] Only three of these embryos survived until birth, and only one survived to adulthood.[29] Millie, the offspring that survived, took 95 attempts to produce.[41] Because the procedure was not automated and had to be performed manually under a microscope, SCNT was very resource intensive. Another reason why there is such high mortality rate with the cloned offspring is due to the fetus being larger than even other large offspring, resulting in death soon after birth.[41] The biochemistry involved in reprogramming the differentiated somatic cell nucleus and activating the recipient egg was also far from understood. Another limitation is trying to use one-cell embryos during the SCNT. When using just one-cell cloned embryos, the experiment has a 65% chance to fail in the process of making morula or blastocyst. The biochemistry also has to be extremely precise, as most late term cloned fetus deaths are the result of inadequate placentation.[41] However, by 2014, researchers were reporting success rates of 70-80% with cloning pigs[42] and in 2016 a Korean company, Sooam Biotech, was reported to be producing 500 cloned embryos a day.[43]
In SCNT, not all of the donor cell's genetic information is transferred, as the donor cell's mitochondria that contain their own mitochondrial DNA are left behind. The resulting hybrid cells retain those mitochondrial structures which originally belonged to the egg. As a consequence, clones such as Dolly that are born from SCNT are not perfect copies of the donor of the nucleus. This fact may also hamper the potential benefits of SCNT-derived tissues and organs for therapy, as there may be an immuno-response to the non-self mtDNA after transplant.
Controversy
Nuclear transfer techniques present a different set of ethical considerations than those associated with the use of other stem cells like embryonic stem cells which are controversial for their requirement to destroy an embryo. These different considerations have led to some individuals and organizations who are not opposed to human embryonic stem cell research to be concerned about, or opposed to, SCNT research.[44][45][46]
One concern is that blastula creation in SCNT-based human stem cell research will lead to the reproductive cloning of humans. Both processes use the same first step: the creation of a nuclear transferred embryo, most likely via SCNT. Those who hold this concern often advocate for strong regulation of SCNT to preclude implantation of any derived products for the intention of human reproduction,[47] or its prohibition.[44]
A second important concern is the appropriate source of the eggs that are needed. SCNT requires human egg cells, which can only be obtained from women. The most common source of these eggs today are eggs that are produced and in excess of the clinical need during IVF treatment. This is a minimally invasive procedure, but it does carry some health risks, such as ovarian hyperstimulation syndrome.
One vision for successful stem cell therapies is to create custom stem cell lines for patients. Each custom stem cell line would consist of a collection of identical stem cells each carrying the patient's own DNA, thus reducing or eliminating any problems with rejection when the stem cells were transplanted for treatment. For example, to treat a man with Parkinson's disease, a cell nucleus from one of his cells would be transplanted by SCNT into an egg cell from an egg donor, creating a unique lineage of stem cells almost identical to the patient's own cells. (There would be differences. For example, the mitochondrial DNA would be the same as that of the egg donor. In comparison, his own cells would carry the mitochondrial DNA of his mother.)
Potentially millions of patients could benefit from stem cell therapy, and each patient would require a large number of donated eggs in order to successfully create a single custom therapeutic stem cell line. Such large numbers of donated eggs would exceed the number of eggs currently left over and available from couples trying to have children through assisted reproductive technology. Therefore, healthy young women would need to be induced to sell eggs to be used in the creation of custom stem cell lines that could then be purchased by the medical industry and sold to patients. It is so far unclear where all these eggs would come from.
Stem cell experts consider it unlikely that such large numbers of human egg donations would occur in a developed country because of the unknown long-term public health effects of treating large numbers of healthy young women with heavy doses of hormones in order to induce hyper-ovulation (ovulating several eggs at once). Although such treatments have been performed for several decades now, the long-term effects have not been studied or declared safe to use on a large scale on otherwise healthy women. Longer-term treatments with much lower doses of hormones are known to increase the rate of cancer decades later. Whether hormone treatments to induce hyper-ovulation could have similar effects is unknown. There are also ethical questions surrounding paying for eggs. In general, marketing body parts is considered unethical and is banned in most countries. Human eggs have been a notable exception to this rule for some time.
To address the problem of creating a human egg market, some stem cell researchers are investigating the possibility of creating artificial eggs. If successful, human egg donations would not be needed to create custom stem cell lines. However, this technology may be a long way off.
Policies regarding human SCNT
SCNT involving human cells is currently legal for research purposes in the United Kingdom, having been incorporated into the Human Fertilisation and Embryology Act 1990.[48][5] Permission must be obtained from the Human Fertilisation and Embryology Authority in order to perform or attempt SCNT.
In the United States, the practice remains legal, as it has not been addressed by federal law.[49] However, in 2002, a moratorium on United States federal funding for SCNT prohibits funding the practice for the purposes of research. Thus, though legal, SCNT cannot be federally funded.[50] American scholars have recently argued that because the product of SCNT is a clone embryo, rather than a human embryo, these policies are morally wrong and should be revised.[51]
In 2003, the United Nations adopted a proposal submitted by Costa Rica, calling on member states to "prohibit all forms of human cloning in as much as they are incompatible with human dignity and the protection of human life."[52] This phrase may include SCNT, depending on interpretation.
The Council of Europe's Convention on Human Rights and Biomedicine and its Additional Protocol to the Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine, on the Prohibition of Cloning Human Being appear to ban SCNT of human beings. Of the Council's 45 member states, the Convention has been signed by 31 and ratified by 18. The Additional Protocol has been signed by 29 member nations and ratified by 14.[53]
See also
- Cloning
- Embryogenesis
- Handmade cloning
- In vitro fertilisation
- Induced stem cells
- New Jersey legislation S1909/A2840
- Rejuvenation
- Stem cell controversy
- Stem cell research
References
- Normile, Dennis (24 January 2018). "These monkey twins are the first primate clones made by the method that developed Dolly". Science. doi:10.1126/science.aat1066. Retrieved 24 January 2018.
- Cyranoski, David (24 January 2018). "First monkeys cloned with technique that made Dolly the sheep – Chinese scientists create cloned primates that could revolutionize studies of human disease". Nature. 553 (7689): 387–388. Bibcode:2018Natur.553..387C. doi:10.1038/d41586-018-01027-z. PMID 29368720.
- Council of Europe, Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine (April 4, 1997, retrieved October 6, 2006); Council of Europe, Additional Protocol to the Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine, on the Prohibition of Cloning Human Being (January 12, 1998, retrieved October 6, 2006)
Further reading
- Wilmut I, Beaujean N, de Sousa PA, et al. (October 2002). "Somatic cell nuclear transfer" (PDF). Nature. 419 (6907): 583–6. Bibcode:2002Natur.419..583W. doi:10.1038/nature01079. PMID 12374931. S2CID 4327096.
- Kikyo N, Wolffe AP (January 2000). "Reprogramming nuclei: insights from cloning, nuclear transfer and heterokaryons". J. Cell Sci. 113. (Pt 1): 11–20. doi:10.1242/jcs.113.1.11. PMID 10591621.
- Tian XC, Kubota C, Enright B, Yang X (November 2003). "Cloning animals by somatic cell nuclear transfer—biological factors". Reprod. Biol. Endocrinol. 1 (1): 98. doi:10.1186/1477-7827-1-98. PMC 521203. PMID 14614770.
- Gurdon JB, Byrne JA, Simonsson S (September 2003). "Nuclear reprogramming and stem cell creation". Proc. Natl. Acad. Sci. U.S.A. 100. Suppl 1 (90001): 11819–22. Bibcode:2003PNAS..10011819G. doi:10.1073/pnas.1834207100. PMC 304092. PMID 12920185.
External links
- Research Cloning: Medical and scientific, legal and ethical aspects
- The Basics: Stem Cells and Public Policy The Century Foundation, June 2005
- Research Cloning Basic Science, Center for Genetics and Society, (last modified October 4, 2004, retrieved October 6, 2006)
- Cloning: present uses and promises National Institutes of Health, Paper giving background information on cloning in general and SCNT from The Office of Science Policy Analysis.
- Nuclear Transfer – Stem Cells or Somatic Cell Nuclear Transfer (SCNT) The International Society for Stem Cell Research
- The Hinxton Group: An International Consortium on Stem Cells, Ethics & Law
https://en.wikipedia.org/wiki/Somatic_cell_nuclear_transfer
https://en.wikipedia.org/wiki/Somatic_cell_nuclear_transfer
https://en.wikipedia.org/wiki/Gene_knockout
https://en.wikipedia.org/wiki/StarLink_corn_recall#StarLink_corn
https://en.wikipedia.org/wiki/MON_863
https://en.wikipedia.org/wiki/MON_810
https://en.wikipedia.org/wiki/Amflora
https://en.wikipedia.org/wiki/Bt_cotton
https://en.wikipedia.org/wiki/Tobacco#Genetic_modification
https://en.wikipedia.org/wiki/Knockout_rat
https://en.wikipedia.org/wiki/Ice-minus_bacteria
https://en.wikipedia.org/wiki/Agrobacterium
https://en.wikipedia.org/wiki/Transduction_(genetics)
https://en.wikipedia.org/wiki/Microinjection
https://en.wikipedia.org/wiki/Transgene
A transgene is a gene that has been transferred naturally, or by any of a number of genetic engineering techniques, from one organism to another. The introduction of a transgene, in a process known as transgenesis, has the potential to change the phenotype of an organism. Transgene describes a segment of DNA containing a gene sequence that has been isolated from one organism and is introduced into a different organism. This non-native segment of DNA may either retain the ability to produce RNA or protein in the transgenic organism or alter the normal function of the transgenic organism's genetic code. In general, the DNA is incorporated into the organism's germ line. For example, in higher vertebrates this can be accomplished by injecting the foreign DNA into the nucleus of a fertilized ovum. This technique is routinely used to introduce human disease genes or other genes of interest into strains of laboratory mice to study the function or pathology involved with that particular gene.
The construction of a transgene requires the assembly of a few main parts. The transgene must contain a promoter, which is a regulatory sequence that will determine where and when the transgene is active, an exon, a protein coding sequence (usually derived from the cDNA for the protein of interest), and a stop sequence. These are typically combined in a bacterial plasmid and the coding sequences are typically chosen from transgenes with previously known functions.[1]
Transgenic or genetically modified organisms, be they bacteria, viruses or fungi, serve many research purposes. Transgenic plants, insects, fish and mammals (including humans) have been bred. Transgenic plants such as corn and soybean have replaced wild strains in agriculture in some countries (e.g. the United States). Transgene escape has been documented for GMO crops since 2001 with persistence and invasiveness. Transgenetic organisms pose ethical questions and may cause biosafety problems.
History
The idea of shaping an organism to fit a specific need isn't a new science. However, until the late 1900s farmers and scientist could breed new strains of a plant or organism only from closely related species, because the DNA had to be compatible for offspring to be able to reproduce another generation.[citation needed]
In the 1970 and 1980s, scientists passed this hurdle by inventing procedures for combining the DNA of two vastly different species with genetic engineering. The organisms produced by these procedures were termed transgenic. Transgenesis is the same as gene therapy in the sense that they both transform cells for a specific purpose. However, they are completely different in their purposes, as gene therapy aims to cure a defect in cells, and transgenesis seeks to produce a genetically modified organism by incorporating the specific transgene into every cell and changing the genome. Transgenesis will therefore change the germ cells, not only the somatic cells, in order to ensure that the transgenes are passed down to the offspring when the organisms reproduce. Transgenes alter the genome by blocking the function of a host gene; they can either replace the host gene with one that codes for a different protein, or introduce an additional gene.[2]
The first transgenic organism was created in 1974 when Annie Chang and Stanley Cohen expressed Staphylococcus aureus genes in Escherichia coli.[3] In 1978, yeast cells were the first eukaryotic organisms to undergo gene transfer.[4] Mouse cells were first transformed in 1979, followed by mouse embryos in 1980. Most of the very first transmutations were performed by microinjection of DNA directly into cells. Scientists were able to develop other methods to perform the transformations, such as incorporating transgenes into retroviruses and then infecting cells, using electroinfusion which takes advantage of an electric current to pass foreign DNA through the cell wall, biolistics which is the procedure of shooting DNA bullets into cells, and also delivering DNA into the egg that has just been fertilized.[5]
The first transgenic animals were only intended for genetic research to study the specific function of a gene, and by 2003, thousands of genes had been studied.
Use in plants
A variety of transgenic plants have been designed for agriculture to produce genetically modified crops, such as corn, soybean, rapeseed oil, cotton, rice and more. As of 2012, these GMO crops were planted on 170 million hectares globally.[6]
Golden rice
One example of a transgenic plant species is golden rice. In 1997,[citation needed] five million children developed xerophthalmia, a medical condition caused by vitamin A deficiency, in Southeast Asia alone.[7] Of those children, a quarter million went blind.[7] To combat this, scientists used biolistics to insert the daffodil phytoene synthase gene into Asia indigenous rice cultivars.[8] The daffodil insertion increased the production of β-carotene.[8] The product was a transgenic rice species rich in vitamin A, called golden rice. Little is known about the impact of golden rice on xerophthalmia because anti-GMO campaigns have prevented the full commercial release of golden rice into agricultural systems in need.[9]
Transgene escape
The escape of genetically-engineered plant genes via hybridization with wild relatives was first discussed and examined in Mexico[10] and Europe in the mid-1990s. There is agreement that escape of transgenes is inevitable, even "some proof that it is happening".[6] Up until 2008 there were few documented cases.[6][11]
Corn
Corn sampled in 2000 from the Sierra Juarez, Oaxaca, Mexico contained a transgenic 35S promoter, while a large sample taken by a different method from the same region in 2003 and 2004 did not. A sample from another region from 2002 also did not, but directed samples taken in 2004 did, suggesting transgene persistence or re-introduction.[12] A 2009 study found recombinant proteins in 3.1% and 1.8% of samples, most commonly in southeast Mexico. Seed and grain import from the United States could explain the frequency and distribution of transgenes in west-central Mexico, but not in the southeast. Also, 5.0% of corn seed lots in Mexican corn stocks expressed recombinant proteins despite the moratorium on GM crops.[13]
Cotton
In 2011, transgenic cotton was found in Mexico among wild cotton, after 15 years of GMO cotton cultivation.[14]
Rapeseed (canola)
Transgenic rapeseed Brassicus napus, hybridized with a native Japanese species Brassica rapa, was found in Japan in 2011[15] after they had been identified 2006 in Québec, Canada.[16] They were persistent over a 6-year study period, without herbicide selection pressure and despite hybridization with the wild form. This was the first report of the introgression—the stable incorporation of genes from one gene pool into another—of an herbicide resistance transgene from Brassica napus into the wild form gene pool.[17]
Creeping bentgrass
Transgenic creeping bentgrass, engineered to be glyphosate-tolerant as "one of the first wind-pollinated, perennial, and highly outcrossing transgenic crops", was planted in 2003 as part of a large (about 160 ha) field trial in central Oregon near Madras, Oregon. In 2004, its pollen was found to have reached wild growing bentgrass populations up to 14 kilometres away. Cross-pollinating Agrostis gigantea was even found at a distance of 21 kilometres.[18] The grower, Scotts Company could not remove all genetically engineered plants, and in 2007, the U.S. Department of Agriculture fined Scotts $500,000 for noncompliance with regulations.[19]
Risk assessment
The long-term monitoring and controlling of a particular transgene has been shown not to be feasible.[20] The European Food Safety Authority published a guidance for risk assessment in 2010.[21]
Use in mice
Genetically modified mice are the most common animal model for transgenic research.[22] Transgenic mice are currently being used to study a variety of diseases including cancer, obesity, heart disease, arthritis, anxiety, and Parkinson’s disease.[23] The two most common types of genetically modified mice are knockout mice and oncomice. Knockout mice are a type of mouse model that uses transgenic insertion to disrupt an existing gene’s expression. In order to create knockout mice, a transgene with the desired sequence is inserted into an isolated mouse blastocyst using electroporation. Then, homologous recombination occurs naturally within some cells, replacing the gene of interest with the designed transgene. Through this process, researchers were able to demonstrate that a transgene can be integrated into the genome of an animal, serve a specific function within the cell, and be passed down to future generations.[24]
Oncomice are another genetically modified mouse species created by inserting transgenes that increase the animal’s vulnerability to cancer. Cancer researchers utilize oncomice to study the profiles of different cancers in order to apply this knowledge to human studies.[24]
Use in Drosophila
Multiple studies have been conducted concerning transgenesis in Drosophila melanogaster, the fruit fly. This organism has been a helpful genetic model for over 100 years, due to its well-understood developmental pattern. The transfer of transgenes into the Drosophila genome has been performed using various techniques, including P element, Cre-loxP, and ΦC31 insertion. The most practiced method used thus far to insert transgenes into the Drosophila genome utilizes P elements. The transposable P elements, also known as transposons, are segments of bacterial DNA that are translocated into the genome, without the presence of a complementary sequence in the host’s genome. P elements are administered in pairs of two, which flank the DNA insertion region of interest. Additionally, P elements often consist of two plasmid components, one known as the P element transposase and the other, the P transposon backbone. The transposase plasmid portion drives the transposition of the P transposon backbone, containing the transgene of interest and often a marker, between the two terminal sites of the transposon. Success of this insertion results in the nonreversible addition of the transgene of interest into the genome. While this method has been proven effective, the insertion sites of the P elements are often uncontrollable, resulting in an unfavorable, random insertion of the transgene into the Drosophila genome.[25]
To improve the location and precision of the transgenic process, an enzyme known as Cre has been introduced. Cre has proven to be a key element in a process known as recombinase-mediated cassette exchange (RMCE). While it has shown to have a lower efficiency of transgenic transformation than the P element transposases, Cre greatly lessens the labor-intensive abundance[clarification needed] of balancing random P insertions. Cre aids in the targeted transgenesis of the DNA gene segment of interest, as it supports the mapping of the transgene insertion sites, known as loxP sites. These sites, unlike P elements, can be specifically inserted to flank a chromosomal segment of interest, aiding in targeted transgenesis. The Cre transposase is important in the catalytic cleavage of the base pairs present at the carefully positioned loxP sites, permitting more specific insertions of the transgenic donor plasmid of interest.[26]
To overcome the limitations and low yields that transposon-mediated and Cre-loxP transformation methods produce, the bacteriophage ΦC31 has recently been utilized. Recent breakthrough studies involve the microinjection of the bacteriophage ΦC31 integrase, which shows improved transgene insertion of large DNA fragments that are unable to be transposed by P elements alone. This method involves the recombination between an attachment (attP) site in the phage and an attachment site in the bacterial host genome (attB). Compared to usual P element transgene insertion methods, ΦC31 integrates the entire transgene vector, including bacterial sequences and antibiotic resistance genes. Unfortunately, the presence of these additional insertions has been found to affect the level and reproducibility of transgene expression.
Use in livestock and aquaculture
One agricultural application is to selectively breed animals for particular traits: Transgenic cattle with an increased muscle phenotype has been produced by overexpressing a short hairpin RNA with homology to the myostatin mRNA using RNA interference.[27] Transgenes are being used to produce milk with high levels of proteins or silk from the milk of goats. Another agricultural application is to selectively breed animals, which are resistant to diseases or animals for biopharmaceutical production.[27]
Future potential
The application of transgenes is a rapidly growing area of molecular biology. As of 2005 it was predicted that in the next two decades, 300,000 lines of transgenic mice will be generated.[28] Researchers have identified many applications for transgenes, particularly in the medical field. Scientists are focusing on the use of transgenes to study the function of the human genome in order to better understand disease, adapting animal organs for transplantation into humans, and the production of pharmaceutical products such as insulin, growth hormone, and blood anti-clotting factors from the milk of transgenic cows.[citation needed]
As of 2004 there were five thousand known genetic diseases, and the potential to treat these diseases using transgenic animals is, perhaps, one of the most promising applications of transgenes. There is a potential to use human gene therapy to replace a mutated gene with an unmutated copy of a transgene in order to treat the genetic disorder. This can be done through the use of Cre-Lox or knockout. Moreover, genetic disorders are being studied through the use of transgenic mice, pigs, rabbits, and rats. Transgenic rabbits have been created to study inherited cardiac arrhythmias, as the rabbit´s heart markedly better resembles the human heart as compared to the mouse.[29][30] More recently, scientists have also begun using transgenic goats to study genetic disorders related to fertility.[31]
Transgenes may be used for xenotransplantation from pig organs. Through the study of xeno-organ rejection, it was found that an acute rejection of the transplanted organ occurs upon the organ's contact with blood from the recipient due to the recognition of foreign antibodies on endothelial cells of the transplanted organ. Scientists have identified the antigen in pigs that causes this reaction, and therefore are able to transplant the organ without immediate rejection by removal of the antigen. However, the antigen begins to be expressed later on, and rejection occurs. Therefore, further research is being conducted.[citation needed] Transgenic microorganisms capable of producing catalytic proteins or enzymes which increase the rate of industrial reactions.
Ethical controversy
Transgene use in humans is currently fraught with issues. Transformation of genes into human cells has not been perfected yet. The most famous example of this involved certain patients developing T-cell leukemia after being treated for X-linked severe combined immunodeficiency (X-SCID).[32] This was attributed to the close proximity of the inserted gene to the LMO2 promoter, which controls the transcription of the LMO2 proto-oncogene.[33]
See also
- Hybrid
- Fusion protein
- Gene pool
- Gene flow
- Introgression
- Nucleic acid hybridization
- Mouse models of breast cancer metastasis
References
- Hacein-Bey-Abina, S.; et al. (17 October 2003). "LMO2-Associated Clonal T Cell Proliferation in Two Patients after Gene Therapy for SCID-X1". Science. 302 (5644): 415–419. Bibcode:2003Sci...302..415H. doi:10.1126/science.1088547. PMID 14564000. S2CID 9100335.
Further reading
- Cyranoski, D (2009). "Newly created transgenic primate may become an alternative disease model to rhesus macaques". Nature. 459 (7246): 492. doi:10.1038/459492a. PMID 19478751.
https://en.wikipedia.org/wiki/Transgene
Category:Gene delivery
This category inlcludes articles on gene delivery, a technique of introducing genetic materials inside cells.
Subcategories
This category has only the following subcategory.
G
- Gene therapy (53 P)
Pages in category "Gene delivery"
The following 29 pages are in this category, out of 29 total. This list may not reflect recent changes.
https://en.wikipedia.org/wiki/Category:Gene_delivery
In biology and genetics, the germline is the population of a multicellular organism's cells that pass on their genetic material to the progeny (offspring). In other words, they are the cells that form the egg, sperm and the fertilised egg. They are usually differentiated to perform this function and segregated in a specific place away from other bodily cells.[1]
As a rule, this passing-on happens via a process of sexual reproduction; typically it is a process that includes systematic changes to the genetic material, changes that arise during recombination, meiosis and fertilization for example. However, there are many exceptions across multicellular organisms, including processes and concepts such as various forms of apomixis, autogamy, automixis, cloning or parthenogenesis.[2][3] The cells of the germline are called germ cells.[4] For example, gametes such as a sperm and an egg are germ cells. So are the cells that divide to produce gametes, called gametocytes, the cells that produce those, called gametogonia, and all the way back to the zygote, the cell from which an individual develops.[4]
In sexually reproducing organisms, cells that are not in the germline are called somatic cells. According to this view, mutations, recombinations and other genetic changes in the germline may be passed to offspring, but a change in a somatic cell will not be.[5] This need not apply to somatically reproducing organisms, such as some Porifera[6] and many plants. For example, many varieties of citrus,[7] plants in the Rosaceae and some in the Asteraceae, such as Taraxacum produce seeds apomictically when somatic diploid cells displace the ovule or early embryo.[8]
In an earlier stage of genetic thinking, there was a clear distinction between germline and somatic cells. For example, August Weismann proposed and pointed out, a germline cell is immortal in the sense that it is part of a lineage that has reproduced indefinitely since the beginning of life and, barring accident, could continue doing so indefinitely.[9] However, it is now known in some detail that this distinction between somatic and germ cells is partly artificial and depends on particular circumstances and internal cellular mechanisms such as telomeres and controls such as the selective application of telomerase in germ cells, stem cells and the like.[10]
Not all multicellular organisms differentiate into somatic and germ lines,[11] but in the absence of specialised technical human intervention practically all but the simplest multicellular structures do so. In such organisms somatic cells tend to be practically totipotent, and for over a century sponge cells have been known to reassemble into new sponges after having been separated by forcing them through a sieve.[6]
Germline can refer to a lineage of cells spanning many generations of individuals—for example, the germline that links any living individual to the hypothetical last universal common ancestor, from which all plants and animals descend.
Evolution
Plants and basal metazoans such as sponges (Porifera) and corals (Anthozoa) do not sequester a distinct germline, generating gametes from multipotent stem cell lineages that also give rise to ordinary somatic tissues. It is therefore likely that germline sequestration first evolved in complex animals with sophisticated body plans, i.e. bilaterians. There are several theories on the origin of the strict germline-soma distinction. Setting aside an isolated germ cell population early in embryogenesis might promote cooperation between the somatic cells of a complex multicellular organism.[12] Another recent theory suggests that early germline sequestration evolved to limit the accumulation of deleterious mutations in mitochondrial genes in complex organisms with high energy requirements and fast mitochondrial mutation rates.[11]
DNA damage, mutation and repair
Reactive oxygen species (ROS) are produced as byproducts of metabolism. In germline cells, ROS are likely a significant cause of DNA damages that, upon DNA replication, lead to mutations. 8-Oxoguanine, an oxidized derivative of guanine, is produced by spontaneous oxidation in the germline cells of mice, and during the cell's DNA replication cause GC to TA transversion mutations.[13] Such mutations occur throughout the mouse chromosomes as well as during different stages of gametogenesis.
The mutation frequencies for cells in different stages of gametogenesis are about 5 to 10-fold lower than in somatic cells both for spermatogenesis[14] and oogenesis.[15] The lower frequencies of mutation in germline cells compared to somatic cells appears to be due to more efficient DNA repair of DNA damages, particularly homologous recombinational repair, during germline meiosis.[16] Among humans, about five percent of live-born offspring have a genetic disorder, and of these, about 20% are due to newly arisen germline mutations.[14]
Epigenetic alterations
Epigenetic alterations of DNA include modifications that affect gene expression, but are not caused by changes in the sequence of bases in DNA. A well-studied example of such an alteration is the methylation of DNA cytosine to form 5-methylcytosine. This usually occurs in the DNA sequence CpG, changing the DNA at the CpG site from CpG to 5-mCpG. Methylation of cytosines in CpG sites in promoter regions of genes can reduce or silence gene expression.[17] About 28 million CpG dinucleotides occur in the human genome,[18] and about 24 million CpG sites in the mouse genome (which is 86% as large as the human genome[19]). In most tissues of mammals, on average, 70% to 80% of CpG cytosines are methylated (forming 5-mCpG).[20]
In the mouse, by days 6.25 to 7.25 after fertilization of an egg by a sperm, cells in the embryo are set aside as primordial germ cells (PGCs). These PGCs will later give rise to germline sperm cells or egg cells. At this point the PGCs have high typical levels of methylation. Then primordial germ cells of the mouse undergo genome-wide DNA demethylation, followed by subsequent new methylation to reset the epigenome in order to form an egg or sperm.[21]
In the mouse, PGCs undergo DNA demethylation in two phases. The first phase, starting at about embryonic day 8.5, occurs during PGC proliferation and migration, and it results in genome-wide loss of methylation, involving almost all genomic sequences. This loss of methylation occurs through passive demethylation due to repression of the major components of the methylation machinery.[21] The second phase occurs during embryonic days 9.5 to 13.5 and causes demethylation of most remaining specific loci, including germline-specific and meiosis-specific genes. This second phase of demethylation is mediated by the TET enzymes TET1 and TET2, which carry out the first step in demethylation by converting 5-mC to 5-hydroxymethylcytosine (5-hmC) during embryonic days 9.5 to 10.5. This is likely followed by replication-dependent dilution during embryonic days 11.5 to 13.5.[22] At embryonic day 13.5, PGC genomes display the lowest level of global DNA methylation of all cells in the life cycle.[21]
In the mouse, the great majority of differentially expressed genes in PGCs from embryonic day 9.5 to 13.5, when most genes are demethylated, are upregulated in both male and female PGCs.[22]
Following erasure of DNA methylation marks in mouse PGCs, male and female germ cells undergo new methylation at different time points during gametogenesis. While undergoing mitotic expansion in the developing gonad, the male germline starts the re-methylation process by embryonic day 14.5. The sperm-specific methylation pattern is maintained during mitotic expansion. DNA methylation levels in primary oocytes before birth remain low, and re-methylation occurs after birth in the oocyte growth phase.[21]
See also
References
- Yamaguchi S, Hong K, Liu R, Inoue A, Shen L, Zhang K, Zhang Y (March 2013). "Dynamics of 5-methylcytosine and 5-hydroxymethylcytosine during germ cell reprogramming". Cell Res. 23 (3): 329–39. doi:10.1038/cr.2013.22. PMC 3587712. PMID 23399596.
https://en.wikipedia.org/wiki/Germline
In botany, apomixis is asexual reproduction without fertilization.[1] Its etymology is Greek for "away from" + "mixing". This definition notably does not mention meiosis. Thus "normal asexual reproduction" of plants, such as propagation from cuttings or leaves, has never been considered to be apomixis, but replacement of the seed by a plantlet or replacement of the flower by bulbils were categorized as types of apomixis. Apomictically produced offspring are genetically identical to the parent plant.
Some authors included all forms of asexual reproduction within apomixis, but that generalization of the term has since died out.[2]
In flowering plants, the term "apomixis" is commonly used in a restricted sense to mean agamospermy, i.e., clonal reproduction through seeds. Although agamospermy could theoretically occur in gymnosperms, it appears to be absent in that group.[2]
Apogamy is a related term that has had various meanings over time. In plants with independent gametophytes (notably ferns), the term is still used interchangeably with "apomixis", and both refer to the formation of sporophytes by parthenogenesis of gametophyte cells.
Male apomixis (paternal apomixis) involves replacement of the genetic material of an egg by the genetic material of the pollen.
https://en.wikipedia.org/wiki/Apomixis
The gametophytes of bryophytes, and less commonly ferns and lycopods can develop a group of cells that grow to look like a sporophyte of the species but with the ploidy level of the gametophyte, a phenomenon known as apogamy. The sporophytes of plants of these groups may also have the ability to form a plant that looks like a gametophyte but with the ploidy level of the sporophyte, a phenomenon known as apospory.[7][8]
See also androgenesis and androclinesis described below, a type of male apomixis that occurs in a conifer, Cupressus dupreziana.
https://en.wikipedia.org/wiki/Apomixis
Ploidy (/ˈplɔɪdi/) is the number of complete sets of chromosomes in a cell, and hence the number of possible alleles for autosomal and pseudoautosomal genes. Sets of chromosomes refer to the number of maternal and paternal chromosome copies, respectively, in each homologous chromosome pair, which chromosomes naturally exist as. Somatic cells, tissues, and individual organisms can be described according to the number of sets of chromosomes present (the "ploidy level"): monoploid (1 set), diploid (2 sets), triploid (3 sets), tetraploid (4 sets), pentaploid (5 sets), hexaploid (6 sets), heptaploid[2] or septaploid[3] (7 sets), etc. The generic term polyploid is often used to describe cells with three or more chromosome sets.[4][5]
https://en.wikipedia.org/wiki/Ploidy
| Oikopleura dioica | |
|---|---|
| |
| Oikopleura dioica | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Subphylum: | Tunicata |
| Class: | Appendicularia |
| Order: | Copelata |
| Family: | Oikopleuridae |
| Genus: | Oikopleura |
| Species: | O. dioica
|
| Binomial name | |
| Oikopleura dioica Fol, 1872[1]
| |
| Synonyms | |
| |
Oikopleura dioica is a species of small pelagic tunicate found in the surface waters of most of the world's oceans. It is used as a model organism in research into developmental biology.
Description
Oikopleura dioica is a bioluminescent species.[2] Like other Oikopleuridans, O. dioica have a discrete body and tail as adults and retain their notochord throughout life. They resemble tadpoles in appearance with a body typically between 0.5 and 1 mm (0.02 and 0.04 in) long and a tail about four times that length.
Its body is ovoid and the tail slender. There are two sub-chordal cells outside the central core of muscle in the tail, which are easily observable some half to two thirds of the way down the length of the tail. The mouth has a small lower lip and the buccal glands are small and globular. The endostyle is large, extending nearly as far as the anus. The right lobe of the stomach forms a sac behind the entrance to the intestine. O. dioica sexes are separate, unlike in all other known appendicularians, and the ovary or testes are at the rear of the body.[3]
Distribution
Oikopleura dioica is widely distributed over the continental shelf in tropical and temperate waters in all the world's oceans.[3] It is very abundant in surface waters but in colder seas is replaced by Oikopleura vanhoeffeni and Oikopleura labradoriensis.[4]
Behaviour
Every three or four hours, Oikopleura dioica creates a mucus net "house" which surrounds its body. Water is pumped through this house and minute food particles are filtered out of the water and then transferred into the mouth. Once the gelatinous net "houses" are too clogged to allow further filtration, they are then abandoned and drift down through the water to the seabed as "marine snow".
Use in research
Oikopleura dioica is used as a model organism, a role for which it has several features to recommend it. It has the typical chordate body plan, it is simple to keep and breed in the laboratory, it produces large numbers of eggs and the generation time is only four days at 20 °C (68 °F). The body is also transparent, making it easier to study, and at hatching only consist of 550 cells.[5] The genome has been sequenced and contains about 15,000 genes, approximately half the number occurring in vertebrates. All central Hox genes have been lost.[6] Comparison of the genome with that of other chordates will help identify the genes which appeared early in the vertebrate lineage.
In the Sars International Centre for Marine Molecular Biology, inbred lines have been developed using repeated matings of closely related individuals. The molecular base of a number of aspects of vertebrate development is identical in these simple chordates and in higher vertebrates. As an example, the brachyury gene and the homolog of the PAX2 gene both play a similar role in the development of tunicates as they do in vertebrates. Complex aspects of vertebral development such as the differentiation of the central nervous system can thus be studied in the laboratory.
References
External links
https://en.wikipedia.org/wiki/Oikopleura_dioica
The Southern red muntjac (Muntiacus muntjak) is a deer species native to Southeast Asia. It was formerly known as the Indian muntjac or the common muntjac before the species was taxonomically revised to represent only populations of Sunda and perhaps Malaysia. The other populations being attributed to this species are now attributed to Muntiacus vaginalis (Northern red muntjac). Muntjacs are also referred to as barking deer. It is listed as Least Concern on the IUCN Red List.[1]
This muntjac has soft, short, brownish or grayish hair, sometimes with creamy markings. It is among the smallest deer species. It is an omnivore and eats grass, fruit, shoots, seeds, bird eggs, and small animals, and occasionally scavenges on carrion. Its calls sound like barking, often when frightened by a predator, hence the common name "barking deer". Males have canines, short antlers that usually branch just once near the base, and a large postorbital scent gland used to mark territories.[2]
Name
The species was formerly classified as Cervus muntjac.[3]
Characteristics
The Southern red muntjac has a short but very soft, thick, dense coat that is more dense in cooler regions. Its face is darker and the limbs are dark to reddish brown and the coat color seasonally varies from darker brown to yellowish and grayish brown and is white ventrally. Its ears have much less hair, but otherwise are the same color as the rest of the head. Male muntjacs have short antlers, about 10 cm (3.9 in) long, that protrude from long body hair-covered pedicels above the eyes. Females have tufts of fur and small bony knobs instead of antlers. Males also have elongated (2–4 cm (0.79–1.57 in)), slightly curved upper canines, which can be used in male-male conflicts and inflict serious injury. The body length of muntjacs varies from 89–135 cm (35–53 in), with a 13 to 23 cm (5.1 to 9.1 in) long tail, and shoulder height ranging from 40 to 65 cm (16 to 26 in). Adult weight ranges between 13 and 35 kg (29 and 77 lb),[4][5] with males being larger than females. Muntjacs are unique among the deer, having large, obvious facial (preorbital, in front of the eyes) scent glands used to mark territories or to attract females. Males have larger glands than females.[6]
Distribution and habitat
The southern red muntjac (previously known as the common muntjac) is one the least known mammals of Southeast Asia. It is found in the Malay Peninsula, Sumatra, Java, Bali and Borneo.[1] It is also assumed to be present in peninsular Thailand and southwestern Myanmar.[1] It is extinct in Singapore.[1]M. muntjak is a terrestrial mammal that live in forests and is resilient to changes in its habitat. [1]
Distribution of subspecies (MSW3)
There were 15 subspecies included under the species in MSW3 :[7]
- M. m. annamensis, Indochina
- M. m. aureus, peninsular India
- M. m. bancanus, Belitung and Bangka Islands
- M. m. curvostylis, Thailand
- M. m. grandicornis, Burmese muntjac, Burma
- M. m. malabaricus, South India and Sri Lanka
- M. m. montanus, Sumatran or mountain muntjac, Sumatra
- M. m. muntjak, Javan muntjac, Java and south Sumatra
- M. m. nainggolani, Bali and Lombok Islands
- M. m. nigripes, black-footed or black-legged muntjac, Vietnam and Hainan Island
- M. m. peninsulae, Malaysia
- M. m. pleicharicus, South Borneo
- M. m. robinsoni, Bintan Island and Lingga Islands
- M. m. rubidus, north Borneo
- M. m. vaginalis, Burma to southwest China
Distribution of subspecies (IUCN and MDD)
1-2 of them have since been elevated to species status : M. malabaricus and M. vaginalis (northern red muntjac).[8][9][10]
The subspecies bancanus, montanus, muntjak, nainggolani, peninsulae, pleiharicus, robinsoni, rubidus are included in the southern red muntjak (M. muntjak), while annamensis, aureus, curvostylis, grandicornis, nigripes are included in the northern red muntjac (M. vaginalis).[11]
Ecology and behavior
The Southern red muntjac is also called "barking deer" due to the bark-like sound that it makes as an alarm when danger is present. Other than during the rut (mating season) and for the first six months after giving birth, the adult muntjac is a solitary animal. Adult males in particular are well spaced and marking grass and bushes with secretions from their preorbital glands appears to be involved in the acquisition and maintenance of territory.[12] Males acquire territories that they mark with scent markers by rubbing their preorbital glands (located on their face, just below the eyes) on the ground and on trees, scraping their hooves against the ground, and scraping the bark of trees with their lower incisors. These scent markers allow other muntjacs to know whether a territory is occupied or not. Males often fight with each other over these territories, sufficient vegetation, and for primary preference over females when mating using their short antlers and an even more dangerous weapon, their canines. If a male is not strong enough to acquire his own territory, it will most likely to fall victim to a predator. During the time of the rut, territorial lines are temporarily disregarded and overlap, while males roam constantly in search of a receptive female.
Predators of these deer include tigers, leopards, clouded leopards, pythons, crocodiles, dholes, Asiatic black bears, fishing cats, Asian golden cats and golden jackals.[5] Foxes, raptors and wild boars prey on fawns. They are highly alert creatures. When put into a stressful situation or if a predator is sensed, muntjacs begin making a bark-like sound. Barking was originally thought of as a means of communication between the deer during mating season, as well as an alert.
Reproduction
The Southern red muntjacs are polygamous animals. Females become sexually mature during their first to second year of life. These females are polyestrous, with each cycle lasting about 14 to 21 days and an estrus lasting for 2 days. The gestation period is 6–7 months and they usually bear one offspring at a time, but sometimes produce twins. Females usually give birth in dense growth so that they are hidden from the rest of the herd and predators. The young leaves its mother after about 6 months to establish its own territory. Males often fight between one another for possession of a harem of females. Muntjacs are distinguished from other even-toed ungulates in showing no evidence of a specific breeding season within the species. Adults exhibit relatively large home range overlap both intersexually and intrasexually, meaning that strict territorialism did not occur but some form of site-specific dominance exists.[13]
Evolution and genetics
Paleontological evidence proves that Southern red muntjacs have been around since the late Pleistocene epoch at least 12,000 years ago. Scientists are interested in studying muntjacs because between species, they have a wide variation in number of chromosomes; in fact, the southern red muntjac has the lowest recorded number of chromosomes of any mammal, with males having a diploid number of 7 and females having 6 chromosomes. They are the oldest known members of the deer family, and the earliest known deer-like creatures had horns instead of antlers, but the muntjac is the earliest known species to actually have antlers. Ancestor to muntjacs is the Dicrocerus elegans, which is the oldest known deer to shed antlers. Other fossils found that deer species experienced a split of the Cervinae from the Muntiacinae, the latter of which remained of similar morphology. Muntjacs of this time during the Miocene were smaller than their modern counterparts. Molecular data have suggested that Southern red muntjacs and Fea's muntjacs share a common ancestor, while giant muntjacs are more closely related to Reeve's muntjac. Although the muntjac deer has a long lineage, little has been studied in terms of their fossil record.[14] The female Southern red muntjac deer is the mammal with the lowest recorded diploid number of chromosomes, where 2n = 6.[15] The male has a diploid number of seven chromosomes. In comparison, the similar Reeves's muntjac (M. reevesi) has a diploid number of 46 chromosomes.[14]
Threats
Most muntjacs species have played a major role in Southeastern Asia, being hunted for sport and for their meat and skin. Often, these animals are hunted around the outskirts of agricultural areas, as they are considered a nuisance for damaging crops and ripping bark from trees.
References
- Kinnear, J. F. (2006). "Nature of Biology". Chromosomes: How Many? (3 ed.). Milton, Queensland: John Wiley & Sons Australia Ltd. ISBN 9780731402366.
Further reading
- Hutchins, M., ed. (2004). "Muntjacs". Grzimek's Animal Life Encyclopedia. Vol. 15 (2 ed.). Detroit: The Gale Group Inc.
- Kurt, F. (1990). "Muntjac Deer". Grzimek's Encyclopedia of Mammals. Vol. 5 (1 ed.). St. Louis: McGraw-Hill.
- Nowak, R.M. (1999). "Muntjacs, or Barking Deer". Walker's Mammals of the World. Vol. 2 (6 ed.). Baltimore: The Johns Hopkins University Press.
https://en.wikipedia.org/wiki/Southern_red_muntjac
https://en.wikipedia.org/wiki/Sterkiella_histriomuscorum
https://en.wikipedia.org/wiki/Tetrahymena_thermophila
https://en.wikipedia.org/wiki/Ophioglossum_reticulatum
https://en.wikipedia.org/wiki/Morus_nigra
https://en.wikipedia.org/wiki/Equisetum_arvense
https://en.wikipedia.org/wiki/Botrypus
https://en.wikipedia.org/wiki/Northern_lamprey
https://en.wikipedia.org/wiki/Adansonia_digitata
https://en.wikipedia.org/wiki/Gymnocarpium_robertianum
https://en.wikipedia.org/wiki/American_paddlefish
https://en.wikipedia.org/wiki/Walking_catfish
https://en.wikipedia.org/wiki/Plains_viscacha_rat
https://en.wikipedia.org/wiki/Crucian_carp
https://en.wikipedia.org/wiki/Aquatic_rat
https://en.wikipedia.org/wiki/Shrimp
https://en.wikipedia.org/wiki/Sceptridium
https://en.wikipedia.org/wiki/Great_white_shark
https://en.wikipedia.org/wiki/Azure-winged_magpie
https://en.wikipedia.org/wiki/Saccharum_officinarum
https://en.wikipedia.org/wiki/Turkey_(bird)
https://en.wikipedia.org/wiki/Nepenthes
https://en.wikipedia.org/wiki/Solanum_nigrum
https://en.wikipedia.org/wiki/White-tailed_deer
https://en.wikipedia.org/wiki/Roadside_hawk
https://en.wikipedia.org/wiki/Gray_fox
https://en.wikipedia.org/wiki/Nine-banded_armadillo
https://en.wikipedia.org/wiki/Chinchilla
https://en.wikipedia.org/wiki/Echidna
https://en.wikipedia.org/wiki/Horse
https://en.wikipedia.org/wiki/Mule
https://en.wikipedia.org/wiki/Donkey
https://en.wikipedia.org/wiki/Lymantria_dispar_dispar
https://en.wikipedia.org/wiki/Goat
https://en.wikipedia.org/wiki/Woolly_mammoth
https://en.wikipedia.org/wiki/Elephant
https://en.wikipedia.org/wiki/Bombyx_mori
https://en.wikipedia.org/wiki/Sheep
https://en.wikipedia.org/wiki/Gossypium_hirsutum
https://en.wikipedia.org/wiki/Platypus
https://en.wikipedia.org/wiki/Striped_skunk
https://en.wikipedia.org/wiki/Water_buffalo
https://en.wikipedia.org/wiki/Atelerix
https://en.wikipedia.org/wiki/Peromyscus
https://en.wikipedia.org/wiki/Hare
https://en.wikipedia.org/wiki/Potato
https://en.wikipedia.org/wiki/Tobacco
https://en.wikipedia.org/wiki/Parhyale_hawaiensis
https://en.wikipedia.org/wiki/Human
https://en.wikipedia.org/wiki/Reeves%27s_muntjac
https://en.wikipedia.org/wiki/Coffea_arabica
https://en.wikipedia.org/wiki/Aurelia_aurita
https://en.wikipedia.org/wiki/Oat
https://en.wikipedia.org/wiki/Wheat
https://en.wikipedia.org/wiki/Peanut
https://en.wikipedia.org/wiki/Trans-Pecos_rat_snake
https://en.wikipedia.org/wiki/Mouse
https://en.wikipedia.org/wiki/Beech_marten
https://en.wikipedia.org/wiki/Cat
https://en.wikipedia.org/wiki/Coati
https://en.wikipedia.org/wiki/Sable
https://en.wikipedia.org/wiki/Aldrovanda_vesiculosa
https://en.wikipedia.org/wiki/African_clawed_frog
https://en.wikipedia.org/wiki/Earthworm
https://en.wikipedia.org/wiki/Cassava
https://en.wikipedia.org/wiki/Meerkat
https://en.wikipedia.org/wiki/Alfalfa
https://en.wikipedia.org/wiki/Baker%27s_yeast
https://en.wikipedia.org/wiki/Giraffe
https://en.wikipedia.org/wiki/Pill_millipede
https://en.wikipedia.org/wiki/Cimex
https://en.wikipedia.org/wiki/Quercus_suber
https://en.wikipedia.org/wiki/Solanum_dulcamara
https://en.wikipedia.org/wiki/Fagus_sylvatica
https://en.wikipedia.org/wiki/Snail
https://en.wikipedia.org/wiki/Cephalotus
https://en.wikipedia.org/wiki/Western_clawed_frog
https://en.wikipedia.org/wiki/Setaria_viridis
https://en.wikipedia.org/wiki/Schistosoma_mansoni
https://en.wikipedia.org/wiki/Barley
https://en.wikipedia.org/wiki/Rye
https://en.wikipedia.org/wiki/Tasmanian_devil
https://en.wikipedia.org/wiki/Slime_mold
https://en.wikipedia.org/wiki/Caenorhabditis_elegans
https://en.wikipedia.org/wiki/Arabidopsis_thaliana
https://en.wikipedia.org/wiki/Marchantia_polymorpha
https://en.wikipedia.org/wiki/Macrostomum_lignano
https://en.wikipedia.org/wiki/List_of_organisms_by_chromosome_count
| Ciliated protozoa (Oxytricha trifallax) |
16,000[135] |  |
|
Macronuclear "nanochromosomes"; ampliploid. MAC chromosomes × 1900 ploidy level = 2.964 × 107 chromosomes |
|---|
| Ciliated protozoa (Tetrahymena thermophila) |
10 (in micronucleus) |  |
|
50x = 12,500 (in macronucleus, except minichromosomes) 10,000x = 10,000 (macronuclear minichromosomes)[134] |
|
|---|
https://en.wikipedia.org/wiki/List_of_organisms_by_chromosome_count

A pair of white-tailed deer (Odocoileus virginianus) grazing from a tree.
https://en.wikipedia.org/wiki/List_of_organisms_by_chromosome_count#/media/File:White-tailed_deer_(Odocoileus_virginianus)_grazing_-_20050809.jpg
| Spotted skunk (Spilogale x) |
64 |  |
|
|
|
|---|---|---|---|---|---|
| Horse (Equus caballus) |
64 |  |
| Gaur (Bos gaurus) |
56 |  |
|
|
|
|---|---|---|---|---|---|
| Elephant (Elephantidae) |
56 |  |
| Hyrax (Hyracoidea) |
54 |  |

|
Hyraxes were considered to be the closest living relatives of elephants,[86] but sirenians have been found to be more closely related to elephants. |
|---|
| Striped skunk (Mephitis mephitis) |
50 |  |
|---|
| Deer mouse (Peromyscus maniculatus) |
48 |  |
|---|
| Tobacco (Nicotiana tabacum) |
48 |  |
|
Cultivated species N. tabacum is an amphidiploid (2n=4x=48) evolved through the interspecific hybridization of the ancestors of N. sylvestris (2n=2x=24, maternal donor) and N. tomentosiformis (2n=2x=24, paternal donor) about 200,000 years ago.[75] | [75] |
|---|---|---|---|---|---|
| Potato (Solanum tuberosum) |
48 |  |

|
This is for common potato Solanum tuberosum (tetraploid, 2n = 4x = 48). Other cultivated potato species may be diploid (2n = 2x = 24), triploid (2n = 3x = 36), tetraploid (2n = 4x = 48), or pentaploid (2n = 5x = 60).[76] Wild relatives mostly have 2n=24.[22] |
| European rabbit (Oryctolagus cuniculus) |
44 |  |

|
|
|
|---|---|---|---|---|---|
| Eurasian badger (Meles meles) |
44 |  |
|
|
|
| Moon jellyfish (Aurelia aurita) |
44 |  |
|
|
[70] |
| Dolphin (Delphinidae) |
44 |  |
|
|
|
| Arabian coffee (Coffea arabica) |
44 |  |

|
Out of the 103 species in the genus Coffea, arabica coffee is the only tetraploid species (2n = 4x = 44), the remaining species being diploid with 2n = 2x = 22.[71] |
|
| Reeves's muntjac (Muntiacus reevesi) |
46 |  |
|
|
|
| Human (Homo sapiens) |
46 |  |

|
44 autosomal. and 2 allosomic (sex) | [72] |
| Nilgai (Boselaphus tragocamelus) |
46 | 
|
|
|
[73] |
| Parhyale hawaiensis | 46 |  |

|
|
[74] |
| Wheat (Triticum aestivum) |
42 |  |

|
This is a hexaploid with 2n=6x=42. Durum wheat is Triticum turgidum var. durum, and is a tetraploid with 2n=4x=28.[22] | [22] |
|---|---|---|---|---|---|
| Rhesus monkey (Macaca mulatta) |
42 |  |

|
|
[68] |
| Rat (Rattus norvegicus) |
42 |  |

|
|
[69] |
| Oats (Avena sativa) |
42 |  |

|
This is a hexaploid with 2n=6x=42. Diploid and tetraploid cultivated species also exist.[22] | [22] |
|
|
|
| |||
| American beaver (Castor canadensis) |
40 |  |
|
|
|
|---|---|---|---|---|---|
| Peanut (Arachis hypogaea) |
40 |  |

|
Cultivated peanut is an allotetraploid (2n = 4x = 40). Its closest relatives are the diploid (2n = 2x = 20).[67] | [67] |
| Trans-Pecos ratsnake (Bogertophis subocularis) |
40 |  |
|
|
[65] |
|---|---|---|---|---|---|
| Mouse (Mus musculus) |
40 |  |
|
|
[66] |
| Cat (Felis catus) |
38 |  |

|
|
|
|---|---|---|---|---|---|
| Beech marten (Martes foina) |
38 |  |
|
|
|
| Baja California rat snake (Bogertophis rosaliae) |
38 |  |
|
|
[64] |
| American marten (Martes americana) |
38 |  |
|
|
|
| Sea otter (Enhydra lutris) |
38 |  |
|
|
|
|---|---|---|---|---|---|
| Sable (Martes zibellina) |
38 |
|
|
| |
| Raccoon (Procyon lotor) |
38 |  |
|
|
[63] |
| Earthworm (Lumbricus terrestris) |
36 |  |
|
|
|
|---|---|---|---|---|---|
| African clawed frog (Xenopus laevis) |
36 |  |

|
|
[35] |
| Waterwheel plant (Aldrovanda vesiculosa) |
38 |  |
|
|
[36] |
| Meerkat (Suricata suricatta) |
36 |  |
|
|
|
|---|---|---|---|---|---|
| Cassava (Manihot esculenta) |
36 | 
|
|
[62] |
| Starfish (Asteroidea) |
36 |  |
|
|
|
|---|
| Sunflower (Helianthus annuus) |
34 |  |
|
|
[59] |
|---|---|---|---|---|---|
| Porcupine (Erethizon dorsatum) |
34 |  |
|
|
[60] |
| Globe artichoke (Cynara cardunculus var. scolymus) |
34 |  |

|
|
[61] |
| American badger (Taxidea taxus) |
32 |  |
|
|
|
|---|---|---|---|---|---|
| Alfalfa (Medicago sativa) |
32 |  |

|
Cultivated alfalfa is tetraploid, with 2n=4x=32. Wild relatives have 2n=16.[22]: 165 | [22] |
| Baker's yeast (Saccharomyces cerevisiae) |
32 |  |
|
|
|
|---|---|---|---|---|---|
| European honey bee (Apis mellifera) |
32/16 |  |

|
32 for females (2n = 32), males are haploid and thus have 16 (1n =16).[57] | [57] |
| Bed bug (Cimex lectularius) |
29–47 |  |
26 autosomes and varying number of the sex chromosomes from three (X1X2Y) to 21 (X1X2Y+18 extra Xs).[52] | [52] | |
|---|---|---|---|---|---|
| Pill millipede (Arthrosphaera magna attems) |
30 |  |
|
|
[53] |
| Giraffe (Giraffa camelopardalis) |
30 |  |

|
|
[54] |
| American mink (Neogale vison) |
30 |  |
|
|
|---|
| Tomato (Solanum lycopersicum) |
24 |  |

|
|
[44] |
|---|---|---|---|---|---|
| European beech (Fagus sylvatica) |
24 |  |

|
|
[45] |
| Bittersweet nightshade (Solanum dulcamara) |
24 |  |
|
|
[46][47] |
| Cork oak (Quercus suber) |
24 |  |
|
|
[48] |
| Silverleaf nightshade (Solanum elaeagnifolium) |
24 |  |
|
|
[42] |
|---|---|---|---|---|---|
| Sweet chestnut (Castanea sativa) |
24 |  |

|
|
[43] |
| Bean (Phaseolus sp.) |
22 |  |

|
All species in the genus Phaseolus have the same chromosome number, including common bean (P. vulgaris), runner bean (P. coccineus), tepary bean (P. acutifolius) and lima bean (P. lunatus).[22] | [22] |
|---|---|---|---|---|---|
| Snail | 24 |  |
|
|
|
| Cacao (Theobroma cacao) |
20 |  |

|
|
[37] |
|---|---|---|---|---|---|
| Eucalyptus (Eucalyptus) |
22 |  |
Although some contradictory cases have been reported, the large homogeneity of the chromosome number 2n = 22 is now known for 135 (33.5%) distinct species among genus Eucalyptus.[38] | [39] | |
| Virginia opossum (Didelphis virginiana) |
22 | 
|
|
[40] |
| Maize (Zea mays) |
20 |  |
|
[22] | |
|---|---|---|---|---|---|
| Cannabis (Cannabis sativa) |
20 |  |
|
| |
| Western clawed frog (Xenopus tropicalis) |
20 |  |

|
|
[35] |
| Australian pitcher plant (Cephalotus follicularis) |
20 |  |
|
|
[36] |
| Setaria viridis (Setaria viridis) |
18 |  |
|
[34] |
|---|
| Itch mite (Sarcoptes scabiei) |
17/18 |  |

|
According to the observation of embryonic cells of egg, chromosome number of the itch mite is either 17 or 18. While the cause for the disparate numbers is unknown, it may arise because of an XO sex determination mechanism, where males (2n=17) lack the sex chromosome and therefore have one less chromosome than the female (2n=18).[29] | [29] |
|---|---|---|---|---|---|
| Radish (Raphanus sativus) |
18 |  |
|
[22] | |
| Carrot (Daucus carota) |
18 |  |

|
The genus Daucus includes around 25 species. D. carota has nine chromosome pairs (2n = 2x = 18). D. capillifolius, D. sahariensis and D. syrticus are the other members of the genus with 2n = 18, whereas D. muricatus (2n = 20) and D. pusillus (2n = 22) have a slightly higher chromosome number. A few polyploid species as for example D. glochidiatus (2n = 4x = 44) and D. montanus (2n = 6x = 66) also exist.[30] | [30] |
| Cabbage (Brassica oleracea) |
18 |  |

|
Broccoli, cabbage, kale, kohlrabi, brussels sprouts, and cauliflower are all the same species and have the same chromosome number.[22] | [22] |
| Citrus (Citrus) |
18 |  |
Chromosome number of the genus Citrus, which including lemons, oranges, grapefruit, pomelo and limes, is 2n = 18.[31] | [32] |
| Kangaroo | 16 |  |
This includes several members of genus Macropus, but not the red kangaroo (M. rufus, 20) | [25] | |
|---|---|---|---|---|---|
| Botryllus schlosseri | 16 |  |
|
|
[26] |
| Schistosoma mansoni | 16 |  |

|
2n=16. 7 autosomal pairs and ZW sex-determination pair.[27] | [27] |
| Welsh onion (Allium fistulosum) |
16 |  |

|
|
[28] |
| Garlic (Allium sativum) |
16 |  |

|
|
[28] |
| Cucumber (Cucumis sativus) |
14 |  |
|
[21] | |
|---|---|---|---|---|---|
| Tasmanian devil (Sarcophilus harrisii) |
14 |  |

|
|
|
| Rye (Secale cereale) |
14 |  |
|
[22] | |
| Pea (Pisum sativum) |
14 |  |

|
|
[22] |
| Barley (Hordeum vulgare) |
14 |  |

|
|
[23] |
| Aloe vera | 14 |  |

|
The diploid chromosome number is 2n = 14 with four pair of long acrocentric chromosomes ranging from 14.4 μm to 17.9 μm and three pair of short sub metacentric chromosomes ranging from 4.6 μm to 5.4 μm.[24] | [24] |
|
| |||||
| Slime mold (Dictyostelium discoideum) |
12 |  |
|
|
[20] |
|---|
| Australian daisy (Brachyscome dichromosomatica) |
12 |  |
|
This species can have more B chromosomes than A chromosomes at times, but 2n=4. | [16] |
|---|---|---|---|---|---|
| Nematode (Caenorhabditis elegans) |
12/11 | 
|
12 for hermaphrodites, 11 for males |
| |
| Spinach (Spinacia oleracea) |
12 |  |

|
|
[17] |
| Macrostomum lignano | 8 | 
|
[13] |
| |
|---|---|---|---|---|---|
| Marchantia polymorpha | 9 |  |

|
Typically haploid with dominant gametophyte stage. 8 autosomes and 1 allosome (sex chromosome). The sex-determination system used by this species and most other bryophytes is called UV. Spores can carry either the U chromosome, which results in female gametophytes, or the V chromosome, which results in males. The chromosome number n = 9 is the basic number in many species of Marchantiales. In some species of Marchantiales, plants with various ploidy levels (having 18 or 27 chromosomes) were reported, but this is rare in nature. | [14] |
| Thale cress (Arabidopsis thaliana) |
10 |  |

|
|
|
| Thale cress (Arabidopsis thaliana) |
10 |  |

|
|
|
|---|
| Hieracium | 8 |  |
|
|
|
|---|---|---|---|---|---|
| Fruit fly (Drosophila melanogaster) |
8 |  |

|
6 autosomal and 2 allosomic (sex) | [12] |
| Macrostomum lignano | 8 | 
|
[13] |
| Yellow fever mosquito (Aedes aegypti) |
6 |  |

|
The 2n=6 chromosome number is conserved in the entire family Culicidae, except in Chagasia bathana, which has 2n=8.[9] | [9] |
|---|---|---|---|---|---|
| Indian muntjac (Muntiacus muntjak) |
6/7 |  |

|
2n = 6 for females and 7 for males. The lowest diploid chromosomal number in mammals.[10] | [11] |
| Oikopleura dioica | 6 |  |
|
|
[8] |
|---|
| Jack jumper ant (Myrmecia pilosula) |
2/1 |  |
|
2 for females, males are haploid and thus have 1; smallest number possible. Other ant species have more chromosomes.[5] | [5] |
|---|---|---|---|---|---|
| Spider mite (Tetranychidae) |
4–14 |  |
|
Spider mites (family Tetranychidae) are typically haplodiploid (males are haploid, while females are diploid)[6] | [6] |
| Cricotopus sylvestris | 4 |
|
|
[7] |
Cytogenetics is essentially a branch of genetics, but is also a part of cell biology/cytology (a subdivision of human anatomy), that is concerned with how the chromosomes relate to cell behaviour, particularly to their behaviour during mitosis and meiosis.[1] Techniques used include karyotyping, analysis of G-banded chromosomes, other cytogenetic banding techniques, as well as molecular cytogenetics such as fluorescence in situ hybridization (FISH) and comparative genomic hybridization (CGH).
https://en.wikipedia.org/wiki/Cytogenetics
| Walking catfish | |
|---|---|

| |
| Specimen from Jatimulyo, Lumajang Regency. | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Actinopterygii |
| Order: | Siluriformes |
| Family: | Clariidae |
| Genus: | Clarias |
| Species: | C. batrachus
|
| Binomial name | |
| Clarias batrachus | |
| Synonyms | |
| |
The walking catfish (Clarias batrachus) is a species of freshwater airbreathing catfish native to Southeast Asia. It is named for its ability to "walk" and wiggle across dry land, to find food or suitable environments. While it does not truly walk as most bipeds or quadrupeds do, it has the ability to use its pectoral fins to keep it upright as it makes a wiggling motion with snakelike movements to traverse land.[2] This fish normally lives in slow-moving and often stagnant waters in ponds, swamps, streams, and rivers, as well as in flooded rice paddies, or temporary pools that may dry up. When this happens, its "walking" skill allows the fish to move to other aquatic environments. Considerable taxonomic confusion surrounds this species and it has frequently been confused with other close relatives.[3][4] One main distinction between the walking catfish and the native North American ictalurid catfish with which it sometimes is confused, is that the walking catfish lacks an adipose fin.[5]
https://en.wikipedia.org/wiki/Walking_catfish
| African wild dog | |
|---|---|

| |
| African wild dog in Tswalu Kalahari Reserve, South Africa | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Carnivora |
| Family: | Canidae |
| Subfamily: | Caninae |
| Tribe: | Canini |
| Genus: | Lycaon |
| Species: | L. pictus
|
| Binomial name | |
| Lycaon pictus | |

| |
| African wild dog range according to the IUCN.
Extant (resident)
Probably extant (resident)
| |
The African wild dog (Lycaon pictus), also called the painted dog or Cape hunting dog, is a wild canine which is a native species to sub-Saharan Africa. It is the largest wild canine in Africa, and the only extant member of the genus Lycaon, which is distinguished from Canis by dentition highly specialised for a hypercarnivorous diet, and by a lack of dewclaws. It is estimated that about 6,600 adults (including 1,400 mature individuals) live in 39 subpopulations that are all threatened by habitat fragmentation, human persecution, and outbreaks of disease. As the largest subpopulation probably comprises fewer than 250 individuals, the African wild dog has been listed as endangered on the IUCN Red List since 1990.[2]
The species is a specialised diurnal hunter of antelopes, which it catches by chasing them to exhaustion. Its natural enemies are lions and spotted hyenas: the former will kill the dogs where possible, whilst hyenas are frequent kleptoparasites.[4]
Like other canids, the African wild dog regurgitates food for its young, but also extends this action to adults, as a central part of the pack's social life.[5][6][7] The young are allowed to feed first on carcasses.
The African wild dog has been respected in several hunter-gatherer societies, particularly those of the San people and Prehistoric Egypt.
https://en.wikipedia.org/wiki/African_wild_dog
The list of organisms by chromosome count describes ploidy or numbers of chromosomes in the cells of various plants, animals, protists, and other living organisms. This number, along with the visual appearance of the chromosome, is known as the karyotype,[1][2][3] and can be found by looking at the chromosomes through a microscope. Attention is paid to their length, the position of the centromeres, banding pattern, any differences between the sex chromosomes, and any other physical characteristics.[4] The preparation and study of karyotypes is part of cytogenetics.
https://en.wikipedia.org/wiki/List_of_organisms_by_chromosome_count
| Part of a series on |
| Sex |
|---|
 |
| Biological terms |
| Sexual reproduction |
| Sexuality |
| Sexual system |
The XO sex-determination system (sometimes X0 sex-determination system) is a system that some species of insects, arachnids, and mammals use to determine the sex of offspring. In this system, there is only one sex chromosome, referred to as X. Males only have one X chromosome (XO), while females have two (XX). The letter O (sometimes a zero) signifies the lack of a second X. Maternal gametes always contain an X chromosome, so the sex of the animals' offspring depends on whether a sex chromosome is present in the male gamete. Its sperm normally contains either one X chromosome or no sex chromosomes at all.
This system determines the sex of offspring among:
- Most arachnids[1] with the exception of mites where a small majority are haplodiploid,[2]
- Almost all apterygote and Paleopteran insects (e.g., dragonflies, silverfish)
- Most exopterygote insects (e.g., grasshoppers, crickets, cockroaches)
- Some nematodes,[1] crustaceans,[1] gastropod molluscs,[3] and bony fish,[4] notably in the genus Ancistrus[5]
- Several mammals
- A few species of bat, including the hammer-headed bat,[6] Buettikofer's epauletted fruit bat, Franquet's epauletted fruit bat, Peters's epauletted fruit bat, and Gambian epauletted fruit bat[7]
- The Ryukyu spiny rat and Tokunoshima spiny rat[8]
In a variant of this system, most individuals have two sex chromosomes (XX) and are hermaphroditic, producing both eggs and sperm with which they can fertilize themselves, while rare individuals are male and have only one sex chromosome (XO). The model organism Caenorhabditis elegans—a nematode frequently used in biological research—is one such organism.
Some Drosophila species have XO males.[9] These are thought to arise via the loss of the Y chromosome.[original research]
Evolution
XO sex determination can evolve from XY sex determination with about 2 million years.[10]
Parthenogenesis
Parthenogenesis with XO sex-determination can occur by different mechanisms to produce either male or female offspring.[11]
See also
- Sex-determination system
- Sexual differentiation
- Haplodiploid sex-determination system
- XY sex-determination system
- ZO sex-determination system
- ZW sex-determination system
- Temperature-dependent sex determination
- X chromosome
- Y chromosome
References
- Hales, Dinah F.; Alex C. C. Wilson; Mathew A. Sloane; Jean-Christophe Simon; Jean-François Legallic; Paul Sunnucks (2002). "Lack of Detectable Genetic Recombination on the X Chromosome During the Parthenogenetic Production of Female and Male Aphids". Genetics Research. 79 (3): 203–209. doi:10.1017/S0016672302005657. PMID 12220127.
https://en.wikipedia.org/wiki/XO_sex-determination_system
| Snails | |
|---|---|

| |
| Helix pomatia, a species of land snail | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Mollusca |
| Class: | Gastropoda |
A snail is a shelled gastropod. The name is most often applied to land snails, terrestrial pulmonate gastropod molluscs. However, the common name snail is also used for most of the members of the molluscan class Gastropoda that have a coiled shell that is large enough for the animal to retract completely into. When the word "snail" is used in this most general sense, it includes not just land snails but also numerous species of sea snails and freshwater snails. Gastropods that naturally lack a shell, or have only an internal shell, are mostly called slugs, and land snails that have only a very small shell (that they cannot retract into) are often called semi-slugs.
Snails have considerable human relevance, including as food items, as pests, and as vectors of disease, and their shells are used as decorative objects and are incorporated into jewelry.[1] The snail has also had some cultural significance, tending to be associated with lethargy. The snail has also been used as a figure of speech in reference to slow-moving things. The snail is the same or similar shape as the cochlea.[2]
https://en.wikipedia.org/wiki/Snail
https://en.wikipedia.org/wiki/Rice
| Rice (Oryza sativa) |
24 |  |

|
|
[22] |
|---|
| Solanum elaeagnifolium | |
|---|---|

| |
| Plant with flowers, unripe berries (green with stripes, center), and previous year's berries (orange, upper left) | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Clade: | Asterids |
| Order: | Solanales |
| Family: | Solanaceae |
| Genus: | Solanum |
| Species: | S. elaeagnifolium
|
| Binomial name | |
| Solanum elaeagnifolium | |
| Synonyms | |
|
See text | |
Solanum elaeagnifolium, the silverleaf nightshade[1] or silver-leaved nightshade, is a common native plant to parts of the southwestern USA, and sometimes weed of western North America and also found in South America. Other common names include prairie berry, silverleaf nettle, white horsenettle or silver nightshade. In South Africa it is known as silver-leaf bitter-apple or satansbos ("Satan's bush" in Afrikaans). More ambiguous names include "bull-nettle", "horsenettle" and the Spanish "trompillo".[2] The plant is also endemic to the Middle East.[3]
Solanum elaeagnifolium was described by A. J. Cavanilles. The plant described under the same name by W. Herbert and C. L. Willdenow based on E.G. von Steudel is Solanum aethiopicum.
https://en.wikipedia.org/wiki/Solanum_elaeagnifolium
| Alfalfa | |
|---|---|

| |
| Medicago sativa[1] | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Clade: | Rosids |
| Order: | Fabales |
| Family: | Fabaceae |
| Subfamily: | Faboideae |
| Genus: | Medicago |
| Section: | M. sect. Medicago |
| Species: | M. sativa
|
| Binomial name | |
| Medicago sativa | |
| Subspecies | |
| Synonyms[3] | |
|
| |
List |
Alfalfa (/ælˈfælfə/) (Medicago sativa), also called lucerne, is a perennial flowering plant in the legume family Fabaceae. It is cultivated as an important forage crop in many countries around the world. It is used for grazing, hay, and silage, as well as a green manure and cover crop. The name alfalfa is used in North America. The name lucerne is the more commonly used name in the United Kingdom, South Africa, Australia, and New Zealand. The plant superficially resembles clover (a cousin in the same family), especially while young, when trifoliate leaves comprising round leaflets predominate. Later in maturity, leaflets are elongated. It has clusters of small purple flowers followed by fruits spiralled in 2 to 3 turns containing 10–20 seeds. Alfalfa is native to warmer temperate climates. It has been cultivated as livestock fodder since at least the era of the ancient Greeks and Romans.
https://en.wikipedia.org/wiki/Alfalfa
Baker's yeast is the common name for the strains of yeast commonly used in baking bread and other bakery products, serving as a leavening agent which causes the bread to rise (expand and become lighter and softer) by converting the fermentable sugars present in the dough into carbon dioxide and ethanol. Baker's yeast is of the species Saccharomyces cerevisiae,[1] and is the same species (but a different strain) as the kind commonly used in alcoholic fermentation, which is called brewer's yeast or the deactivated form nutritional yeast.[2] Baker's yeast is also a single-cell microorganism found on and around the human body.
The use of steamed or boiled potatoes,[3] water from potato boiling,[4] or sugar in a bread dough provides food for the growth of yeasts; however, too much sugar will dehydrate them.[5] Yeast growth is inhibited by both salt and sugar, but more so by salt than sugar.[6] Some sources say fats, such as butter and eggs, slow down yeast growth;[7] others say the effect of fat on dough remains unclear, presenting evidence that small amounts of fat are beneficial for baked bread volume.[8]
Saccharomyces exiguus (also known as S. minor) is a wild yeast found on plants, grains, and fruits that is occasionally used for baking; however, in general, it is not used in a pure form but comes from being propagated in a sourdough starter.
https://en.wikipedia.org/wiki/Baker%27s_yeast
| Pill millipedes | |
|---|---|

| |
| A giant pill millipede from India. | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Subphylum: | Myriapoda |
| Class: | Diplopoda |
| Subclass: | Chilognatha |
| Infraclass: | Pentazonia |
| Superorder: | Oniscomorpha Pocock, 1887 [1] |
| Orders | |
| Synonyms | |
|
Armadillomorpha Verhoeff, 1915 | |
Pill millipedes are any members of two living (and one extinct) orders of millipedes, often grouped together into a single superorder, Oniscomorpha. The name Oniscomorpha refers to the millipedes' resemblance to certain woodlice (Oniscidea), also called pillbugs or "roly-polies". However, millipedes and woodlice are not closely related (belonging to the subphyla Myriapoda and Crustacea, respectively); rather, this is a case of convergent evolution.
https://en.wikipedia.org/wiki/Pill_millipede
| Cork oak | |
|---|---|

| |
| Cork oak in its natural habitat | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Clade: | Rosids |
| Order: | Fagales |
| Family: | Fagaceae |
| Genus: | Quercus |
| Subgenus: | Quercus subg. Cerris |
| Section: | Quercus sect. Cerris |
| Species: | Q. suber
|
| Binomial name | |
| Quercus suber | |
| |
| Synonyms[2] | |
|
| |
List |
Quercus suber, commonly called the cork oak, is a medium-sized, evergreen oak tree in the section Quercus sect. Cerris. It is the primary source of cork for wine bottle stoppers and other uses, such as cork flooring and as the cores of cricket balls. It is native to southwest Europe and northwest Africa. In the Mediterranean basin the tree is an ancient species with fossil remnants dating back to the Tertiary period.[3]
It endures drought and makes little demand on the soil quality and is regarded as a defence against desertification. Cork oak woodlands are home to a multitude of animal and plant species. Since cork is increasingly being displaced by other materials as a bottle cap, these forests are at risk as part of the cultural landscape and animal species such as the Iberian lynx and imperial eagles are threatened with extinction.[4]
https://en.wikipedia.org/wiki/Quercus_suber
| Bittersweet Nightshade | |
|---|---|

| |
| Solanum dulcamara[1] | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Clade: | Asterids |
| Order: | Solanales |
| Family: | Solanaceae |
| Genus: | Solanum |
| Species: | S. dulcamara
|
| Binomial name | |
| Solanum dulcamara | |
Solanum dulcamara is a species of vine in the genus Solanum (which also includes the potato and the tomato) of the family Solanaceae. Common names include bittersweet, bittersweet nightshade, bitter nightshade, blue bindweed, Amara Dulcis,[3] climbing nightshade,[4] felonwort, fellenwort, felonwood, poisonberry, poisonflower, scarlet berry, snakeberry,[5][6][7] trailing bittersweet, trailing nightshade, violet bloom, and woody nightshade.
It is native to Europe and Asia, and widely naturalised elsewhere, including North America.
https://en.wikipedia.org/wiki/Solanum_dulcamara
| Meerkat Temporal range: [1]
| |
|---|---|

| |
| A mob of meerkats in Tswalu Kalahari Reserve | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Carnivora |
| Suborder: | Feliformia |
| Family: | Herpestidae |
| Genus: | Suricata |
| Species: | S. suricatta
|
| Binomial name | |
| Suricata suricatta (Schreber, 1776)
| |
| Subspecies | |
|
| |
List | |

| |
Range of the meerkat[2]
| |
| Synonyms[3][4] | |
|---|---|
|
|
List |
The meerkat (Suricata suricatta) or suricate is a small mongoose found in southern Africa. It is characterised by a broad head, large eyes, a pointed snout, long legs, a thin tapering tail, and a brindled coat pattern. The head-and-body length is around 24–35 cm (9.4–13.8 in), and the weight is typically between 0.62 and 0.97 kg (1.4 and 2.1 lb). The coat is light grey to yellowish-brown with alternate, poorly-defined light and dark bands on the back. Meerkats have foreclaws adapted for digging and have the ability to thermoregulate to survive in their harsh, dry habitat. Three subspecies are recognised.
Meerkats are highly social, and form packs of two to 30 individuals each that occupy home ranges around 5 km2 (1.9 sq mi) in area. There is a social hierarchy—generally dominant individuals in a pack breed and produce offspring, and the nonbreeding, subordinate members provide altruistic care to the pups. Breeding occurs around the year, with peaks during heavy rainfall; after a gestation of 60 to 70 days, a litter of three to seven pups is born.
They live in rock crevices in stony, often calcareous areas, and in large burrow systems in plains. The burrow systems, typically 5 m (16 ft) in diameter with around 15 openings, are large underground networks consisting of two to three levels of tunnels. These tunnels are around 7.5 cm (3.0 in) high at the top and wider below, and extend up to 1.5 m (4 ft 11 in) into the ground. Burrows have moderated internal temperatures and provide a comfortable microclimate that protects meerkats in harsh weather and at extreme temperatures.
Meerkats are active during the day, mostly in the early morning and late afternoon; they remain continually alert and retreat to burrows when sensing danger. They use a broad variety of calls to communicate among one another for different purposes, for example to raise an alarm on sighting a predator. Primarily insectivorous, meerkats feed heavily on beetles and lepidopterans, arthropods, amphibians, small birds, reptiles, and plant material in their diet.
Commonly living in arid, open habitats with little woody vegetation, meerkats occur in southwestern Botswana, western and southern Namibia, and northern and western South Africa; the range barely extends into southwestern Angola. With no significant threats to the population, the meerkat is listed as Least Concern on the IUCN Red List. Meerkats are widely depicted in television, movies and other media.
https://en.wikipedia.org/wiki/Meerkat

| Mango (Mangifera indica) |
40 |  |
|
|
[22] |
|---|
| Wheat (Triticum aestivum) |
42 |  |

|
This is a hexaploid with 2n=6x=42. Durum wheat is Triticum turgidum var. durum, and is a tetraploid with 2n=4x=28.[22] | [22] |
|---|
| Aldrovanda vesiculosa | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Order: | Caryophyllales |
| Family: | Droseraceae |
| Genus: | Aldrovanda |
| Species: | A. vesiculosa
|
| Binomial name | |
| Aldrovanda vesiculosa | |

| |
| Distribution | |
| Synonyms[2] | |
| |
Aldrovanda vesiculosa, commonly known as the waterwheel plant, is the sole extant species in the flowering plant genus Aldrovanda of the family Droseraceae. The plant captures small aquatic invertebrates using traps similar to those of the Venus flytrap. The traps are arranged in whorls around a central, free-floating stem, giving rise to the common name. This is one of the few plant species capable of rapid movement.
While the genus Aldrovanda is now monotypic, up to 19 extinct species are known in the fossil record.[3][4][5] While the species displays a degree of morphological plasticity between populations, A. vesiculosa possesses a very low genetic diversity across its entire range.[5]
A. vesiculosa has declined over the last century to only 50 confirmed extant populations worldwide. These are spread across Europe, Africa, Asia, and Australia.[5] However, potentially invasive populations exist in the eastern United States.[6] It is kept by hobbyists.
https://en.wikipedia.org/wiki/Aldrovanda_vesiculosa
| Rat (Rattus norvegicus) |
42 |  |

|
|
[69] |
|---|
| Upland cotton (Gossypium hirsutum) |
52 |  |

|
This is for the cultivated species G. hirsutum (allotetraploid, 2n=4x=52). This species accounts for 90% of the world cotton production. Among 50 species in the genus Gossypium, 45 are diploid (2n = 2x = 26) and 5 are allotetraploid (2n = 4x = 52).[85] | [85] |
|---|
A frontal view of a rock hyrax at the Serengeti Visitor's Centre in Tanzania.
https://en.wikipedia.org/wiki/List_of_organisms_by_chromosome_count


Bos gaurus
https://en.wikipedia.org/wiki/List_of_organisms_by_chromosome_count#/media/File:Bos_gaurus.jpeg
| Spotted skunk (Spilogale x) |
64 |  |
|
|---|
| Sirenia Temporal range: Early Eocene – Holocene,
| |
|---|---|

| |
| Clockwise from upper left: West Indian manatee, African manatee, Steller's sea cow, dugong, Amazonian manatee | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Superorder: | Afrotheria |
| Clade: | Paenungulata |
| Clade: | Tethytheria |
| Order: | Sirenia Illiger, 1811 |
| Families | |

| |
| West Indian in green, Amazonian in red, African in orange, dugong in blue, Steller's sea cow circled (yellow) | |
| Synonyms[1] | |
| |
The Sirenia (/saɪˈriːniə/), commonly referred to as sea cows or sirenians, are an order of fully aquatic, herbivorous mammals that inhabit swamps, rivers, estuaries, marine wetlands, and coastal marine waters. The extant Sirenia comprise two distinct families: Dugongidae (the dugong and the now extinct Steller's sea cow) and Trichechidae (manatees, namely the Amazonian manatee, West Indian manatee, and West African manatee) with a total of four species.[2] The Protosirenidae (Eocene sirenians) and Prorastomidae (terrestrial sirenians) families are extinct. Sirenians are classified in the clade Paenungulata, alongside the elephants and the hyraxes, and evolved in the Eocene 50 million years ago (mya). The Dugongidae diverged from the Trichechidae in the late Eocene or early Oligocene (30–35 mya).[3][4]
Sirenians grow to between 2.5 and 4 metres (8.2 and 13.1 feet) in length and 1,500 kilograms (3,300 pounds) in weight. The recently extinct Steller's sea cow was the largest known sirenian to have lived, reaching lengths of 10 metres (33 feet) and weights of 5 to 10 tonnes (5.5 to 11.0 short tons).[2]
Sirenians have a large, fusiform body to reduce drag through the water and heavy bones that act as ballast to counteract the buoyancy of their blubber. They have a thin layer of blubber and consequently are sensitive to temperature fluctuations, which cause migrations when water temperatures dip too low. Sirenians are slow-moving, typically coasting at 8 kilometres per hour (5.0 miles per hour), but they can reach 24 kilometres per hour (15 miles per hour) in short bursts. They use their strong lips to pull out seagrasses, consuming 10–15% of their body weight per day.
https://en.wikipedia.org/wiki/Sirenia
| Mule | |
|---|---|

| |
Domesticated
| |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Perissodactyla |
| Family: | Equidae |
| Tribe: | Equini |
| Genus: | Equus |
| Species: | |
https://en.wikipedia.org/wiki/Mule
| Solanum nigrum | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Clade: | Asterids |
| Order: | Solanales |
| Family: | Solanaceae |
| Genus: | Solanum |
| Species: | S. nigrum
|
| Binomial name | |
| Solanum nigrum | |
| Subspecies | |
|
S. nigrum subsp. nigrum | |
Solanum nigrum, the European black nightshade or simply black nightshade or blackberry nightshade,[1] is a species of flowering plant in the genus Solanum, native to Eurasia and introduced in the Americas, Australasia, and South Africa. Ripe berries and cooked leaves of edible strains are used as food in some locales, and plant parts are used as a traditional medicine. A tendency exists in literature to incorrectly refer to many of the other "black nightshade" species as "Solanum nigrum".[2]
Solanum nigrum has been recorded from deposits of the Paleolithic and Mesolithic era of ancient Britain and it is suggested by the botanist and ecologist Edward Salisbury that it was part of the native flora there before Neolithic agriculture emerged.[3] The species was mentioned by Pliny the Elder in the first century AD and by the great herbalists, including Dioscorides.[4] In 1753, Carl Linnaeus described six varieties of Solanum nigrum in Species Plantarum.[5]
https://en.wikipedia.org/wiki/Solanum_nigrum
Tetrapoda
superclass of the first four-limbed vertebrates and their descendants | |||||||||||||||||||||
| Upload media | |||||||||||||||||||||
| Instance of | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subclass of |
| ||||||||||||||||||||
| Start time |
| ||||||||||||||||||||
| Different from | |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| Taxon author | Otto Jaekel, 1909 | ||||||||||||||||||||
| Authority file | |||||||||||||||||||||
https://commons.wikimedia.org/wiki/Category:Tetrapoda
| Atropa belladonna | |
|---|---|

| |
| Illustration from Köhler's Medicinal Plants 1887 | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Clade: | Asterids |
| Order: | Solanales |
| Family: | Solanaceae |
| Genus: | Atropa |
| Species: | A. belladonna
|
| Binomial name | |
| Atropa belladonna | |
Atropa belladonna, commonly known as belladonna or deadly nightshade, is a toxic perennial herbaceous plant in the nightshade family Solanaceae,[1] which also includes tomatoes, potatoes, and eggplant (aubergine). It is native to Europe, North Africa, and Western Asia, including Türkiye. Its distribution extends from Ireland in the west to western Ukraine and the Iranian province of Gilan in the east. It is also naturalised or introduced in some parts of Canada and the United States.
The foliage and berries are extremely toxic when ingested, containing tropane alkaloids.[1][2][3][4] These toxins include atropine, scopolamine, and hyoscyamine, which cause delirium and hallucinations,[1][2][3][5][6] and are also used as pharmaceutical anticholinergics.[1] Tropane alkaloids are of common occurrence not only in the Old World tribes Hyoscyameae (to which the genus Atropa belongs) and Mandragoreae, but also in the New World tribe Datureae - all of which belong to the subfamily Solanoideae of the plant family Solanaceae.[1]
Atropa belladonna has unpredictable effects.[2] The antidote for belladonna poisoning is physostigmine or pilocarpine, the same as for atropine.[7]
https://en.wikipedia.org/wiki/Atropa_belladonna
Black nightshade is a common name for several plants and may refer to:
- Solanum americanum (American black nightshade) of much of North America
- Solanum nigrum (European black nightshade) of Europe
- Solanum ptychanthum (Eastern black nightshade) of the Caribbean region
https://en.wikipedia.org/wiki/Black_nightshade
| Lymantria dispar dispar | |
|---|---|

| |
| Adult female | |

| |
| Adult male | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Lepidoptera |
| Superfamily: | Noctuoidea |
| Family: | Erebidae |
| Genus: | Lymantria |
| Species: | |
| Subspecies: | L. d. dispar
|
| Trinomial name | |
| Lymantria dispar dispar | |
https://en.wikipedia.org/wiki/Lymantria_dispar_dispar
https://commons.wikimedia.org/wiki/Urocyon_cinereoargenteus
Gray Fox | Desert Museum | Tucson |AZ | 2015-10-12at11-41-174












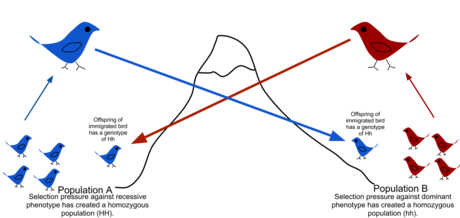





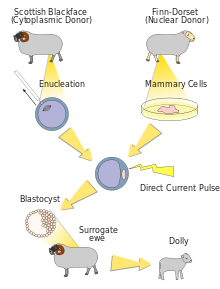
































No comments:
Post a Comment