Granuloma Annulare Lupus erythematosus systemic Lyme Disease Autoimmune Disease
Causes
The condition is usually seen in otherwise healthy people. Occasionally, it may be associated with diabetes or thyroid disease. It has also been associated with auto-immune diseases such as systemic lupus erythematosus, rheumatoid arthritis, Lyme diseaseand Addison's disease. At this time, no conclusive connection has been made between patients.[citation needed]
Pathogenesis
Granuloma annulare is an idiopathic condition, though many catalysts have been proposed. Among these is skin trauma, UV exposure, vaccinations, tuberculin skin testing, and Borrelia and viral infections.[2][4][5]
The mechanisms proposed at a molecular level vary even more. In 1977, Dahl et al. proposed that since the lesions of GA often display a thickening of, occlusion of, or other trauma to blood vessels, blood vessels may be responsible for GA. From their study of 58 patients, they found that immunoglobin M (IgM), complement, and fibrinogen were in the blood vessels of GA areas, suggesting that GA may share similarities with an immune-mediated, type 3 reaction or that chronic immune vasculitis may be involved in the pathogenesis.[6][7] Another study found evidence suggesting blood vessel involvement with masses of intercellular fibrin and thickened basal lamina found around capillaries.[6][8]
Umbert et al. (1976), proposed an alternative pathogenesis: cell-mediated immunity. Their data suggests that lymphokines, such as macrophage-inhibiting factor (MIF), leads to sequestration of macrophages and histiocytes in the dermis. Then, upon lysosomal enzyme release by these sequestered cells, connective tissue damage ensues, which results in GA.[9] Later, these authors found data suggesting that activation of macrophages and fibroblasts are involved in the pathogenesis of GA and that fibrin and the rare IgM and C3 deposition around vessels were more likely a delayed-type hypersensitivity with resulting tissue and vessel changes rather than an immune-complex mediated disease.[10] Further data has been collected supporting this finding.[6][11][12]
https://en.wikipedia.org/wiki/Granuloma_annulare
Lupus, technically known as systemic lupus erythematosus (SLE), is an autoimmune diseasein which the body's immune system mistakenly attacks healthy tissue in many parts of the body.[1]Symptoms vary between people and may be mild to severe.[1] Common symptoms include painful and swollen joints, fever, chest pain, hair loss, mouth ulcers, swollen lymph nodes, feeling tired, and a red rash which is most commonly on the face.[1] Often there are periods of illness, called flares, and periods of remission during which there are few symptoms.[1]
SLE is presumably caused by a genetic susceptibility coupled with an environmental trigger which results in defects in the immune system. One of the factors associated with SLE is vitamin D deficiency.[48]
SLE does run in families, but no single causal gene has been identified. Instead, multiple genes appear to influence a person's chance of developing lupus when triggered by environmental factors. HLA class I, class II, and class III genes are associated with SLE, but only classes I and II contribute independently to increased risk of SLE.[49] Other genes which contain risk variants for SLE are IRF5, PTPN22, STAT4,[50] CDKN1A,[51] ITGAM, BLK,[50] TNFSF4 and BANK1.[52] Some of the susceptibility genes may be population specific.[50] Genetic studies of the rates of disease in families supports the genetic basis of this disease with a heritability of >66%.[53] Identical (monozygotic) twins were found to share susceptibility to the disease at >35% rate compared to fraternal (dizygotic) twins and other full siblings who only showed a 2–5% concordance in shared inheritance.[53]
Since SLE is associated with many genetic regions, it is likely an oligogenic trait, meaning that there are several genes that control susceptibility to the disease.[54]
SLE is regarded as a prototype disease due to the significant overlap in its symptoms with other autoimmune diseases.[55]
https://en.wikipedia.org/wiki/Lupus
https://en.wikipedia.org/wiki/Hypersensitivity_pneumonitis
https://en.wikipedia.org/wiki/Spherocytosis
https://en.wikipedia.org/wiki/Allergic_rhinitis
https://en.wikipedia.org/wiki/Autoimmune_disease
https://en.wikipedia.org/wiki/Immunology
Vaccinia virus (VACV or VV) is a large, complex, enveloped virus belonging to the poxvirus family.[2] It has a linear, double-stranded DNA genome approximately 190 kbp in length, which encodes approximately 250 genes. The dimensions of the virion are roughly 360 × 270 × 250 nm, with a mass of approximately 5–10 fg.[3]The vaccinia virus is the source of the modern smallpox vaccine, which the World Health Organisation used to eradicate smallpox in a global vaccination campaign in 1958–1977. Although smallpox no longer exists in the wild, vaccinia virus is still studied widely by scientists as a tool for gene therapy and genetic engineering.
Smallpox had been an endemic human disease that had a 30% fatality rate. In 1796, the British doctor Edward Jenner proved that an infection with the relatively mild cowpox virus would also confer immunity to the deadly smallpox. Jenner referred to cowpox as variolae vaccinae (smallpox of the cow). However, the origins of the smallpox vaccine became murky over time,[4] especially after Louis Pasteur developed laboratory techniques for creating vaccines in the 19th century. Allan Watt Downie demonstrated in 1939 that the modern smallpox vaccine was serologically distinct from cowpox,[5] and vaccinia was subsequently recognized as a separate viral species. Whole-genome sequencing has revealed that vaccinia is most closely related to horsepox, and the cowpox strains found in Great Britain are the least closely related to vaccinia.[6]
| Vaccinia viru | |
|---|---|
 | |
| A TEM micrograph of Vaccinia virusvirions | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Varidnaviria |
| Kingdom: | Bamfordvirae |
| Phylum: | Nucleocytoviricota |
| Class: | Pokkesviricetes |
| Order: | Chitovirales |
| Family: | Poxviridae |
| Genus: | Orthopoxvirus |
| Species: | Vaccinia viru |
| Member viruses[1] | |
https://en.wikipedia.org/wiki/Vaccinia
Progressive vaccinia is a rare cutaneous condition caused by the vaccinia virus, characterized by painless, but progressive, necrosis and ulceration.[4]
| Progressive vaccinia | |
|---|---|
| Other names | Vaccinia gangrenosum, Vaccinia necrosum or disseminated vaccinia |
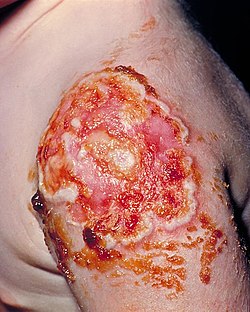 | |
| The patient required a skin graft on her upper left arm in order to correct the necrotic vaccination site, due to the onset of progressive vaccinia, formerly known as vaccinia gangrenosum. | |
| Specialty | Dermatology |
| Symptoms | Malaise, fever, vomiting and tender, enlarged axillary lymph nodes; progresses to septic Pseudomonas aeruginosa, likely from a perirectal abscess, Clostridium difficile (bacteria), Staphylococcus aureus and cell-mediated immunodeficiency. |
| Complications | Necrosis of the injected part, exacerbating to gangrene and eventual amputation. Usually, the pocks tend to go away without scarring; however, the external and internal spread of the virus may have serious consequences in persons with eczema and other forms of atopic dermatitis, in these persons, defects of innate immunity and a high level of Th2 cell activity render the skin unusually permissive to the initiation and rapid spread of vaccinia infection (known as “eczema vaccinatum”)[1][2] |
| Usual onset | 11 days to 6.5 weeks |
| Duration | Long-lasting |
| Causes | Injection by the vaccinia virus(genus: orthopoxvirus) as a countermeasure for smallpox[3] |
| Risk factors | People with cellular immunodeficiencies |
| Diagnostic method | Fever and headache, then progressive ulceration and necrosis of the injection site for smallpox, albeit the lack of inflammation is noted as the "hallmark of PV"[3] |
| Differential diagnosis | May initially be mistaken for leukemia |
| Prevention | Unknown |
| Treatment | Vaccinia Immune Globulin Intravenous (Human) (VIGIV), Emergency Investigational New Drug (E-IND) both administered orally and topically, (in this case ST-246); CMX001, a lipid conjugate of cidofovir and granulocyte colony-stimulating factor for the exiguous normal white blood cells;supportive care; skin graft |
| Medication | Imiquimod, and thiosemicarbazone |
| Prognosis | Lifelong |
| Frequency | every 1 or 2 in a million during routine vaccination during 1963-1968 for smallpox |
| Deaths | fatality rate: 15% |
See also[edit]
- Vaccinia
- Skin lesion
- Necrosis
- Smallpox
- Vaccination
- Chicken pox
- Cowpox- a virus closely related to the vaccinia virus and belongs to the same genus Orthopoxvirus.
Note[edit]
- ^ Immunosuppressed individuals tend to have a larger fatality rate and tendency to get the virus due to HIV infection, iatrogenic immunosuppression, etc. Although these conditions are contraindications to the dermovaccine, inadvertent inoculation after contact with a vaccinee may occur; in layman's terms, inoculation means the introduction of a pathogen or antigen into a living organism to stimulate the production of antibodies.[5] Due to the impaired immune response of the host, the virus multiplies by cell-to-cell spread at the inoculation site, and the lesion expands circumferentially, forming the trademark symptoms called "pocks".
https://en.wikipedia.org/wiki/Progressive_vaccinia
Inoculation for smallpox appears to have started in China around the 1500s.[19][20] Europe adopted this practice from Asia in the first half of the 18th century.[21] In 1796 Edward Jenner introduced the modern smallpox vaccine.[22][23] In 1967, the WHO intensified efforts to eliminate the disease.[10] Smallpox is one of two infectious diseases to have been eradicated, the other being rinderpest in 2011.[24][25] The term "smallpox" was first used in Britain in the early 16th century to distinguish the disease from syphilis, which was then known as the "great pox".[26][27] Other historical names for the disease include pox, speckled monster, and red plague.[3][4][27]
https://en.wikipedia.org/wiki/Smallpox
Rinderpest (also cattle plague or steppe murrain) was an infectious viral disease of cattle, domestic buffalo, and many other species of even-toed ungulates, including gaurs, buffaloes, large antelope, deer, giraffes, wildebeests, and warthogs.[2] The disease was characterized by fever, oral erosions, diarrhea, lymphoid necrosis, and high mortality. Death rates during outbreaks were usually extremely high, approaching 100% in immunologically naïve populations.[3] Rinderpest was mainly transmitted by direct contact and by drinking contaminated water, although it could also be transmitted by air.[4] After a global eradication campaign since the mid-20th century, the last confirmed case of rinderpest was diagnosed in 2001.[5]
https://en.wikipedia.org/wiki/Rinderpest
No comments:
Post a Comment