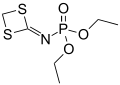
Phosphoramidates (sometimes also called amidophosphates) are a class of phosphorus compounds structurally related to phosphates (or organophosphates) via the substitution of an OR for a NR2. They are derivatives of phosphoramidic acids O=P(OH)(NR2)2, O=P(OH)2(NR2).
A phosphorodiamidate (or diamidophosphate) is a phosphate that has two of its OH groups substituted by NR2 groups to give a species with the general formula O=P(OH)(NH2)2. The substitution of all three OH groups gives the phosphoric triamides (O=P(NR2)3), which are commonly referred to as phosphoramides.[1]
Two examples of natural phosphoramidates are phosphocreatine and the phosphoramidate formed when histidine residues in histidine kinases are phosphorylated.[2] An example of a phosphorodiamidate is morpholino which is used in molecular biology.
https://en.wikipedia.org/wiki/Phosphoramidate
A phosphoramidite (RO)2PNR2 is a monoamide of a phosphite diester. The key feature of phosphoramidites is their markedly high reactivity towards nucleophiles catalyzed by weak acids e.c., triethylammonium chloride or 1H-tetrazole. In these reactions, the incoming nucleophile replaces the NR2 moiety.
Applications[edit]
Nucleoside phosphoramidites[edit]
Phosphoramidites derived from protected nucleosides are referred to as nucleoside phosphoramidites and are widely used in chemical synthesis of DNA, RNA, and other nucleic acids and their analogs.
As ligands[edit]
Certain phosphoramidites are also used as monodentate chiral ligands in asymmetric synthesis.[1] A large group of such ligands is derived from the chiral diol BINOL and can be synthesised by reaction of BINOL with phosphorus trichloride to the chlorophosphite and then reaction with simple secondary amines.[2] This type of ligand was first used in 1996 in an asymmetric copper-catalysed addition of dialkylzincs to enones [3][4]
https://en.wikipedia.org/wiki/Phosphoramidite

No comments:
Post a Comment