n-Butyllithium C4H9Li (abbreviated n-BuLi) is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene (SBS). Also, it is broadly employed as a strong base (superbase) in the synthesis of organic compounds as in the pharmaceutical industry.
Butyllithium is commercially available as solutions (15%, 25%, 1.5 M, 2 M, 2.5 M, 10 M, etc.) in alkanes such as pentane, hexanes, and heptanes. Solutions in diethyl ether and THF can be prepared, but are not stable enough for storage. Annual worldwide production and consumption of butyllithium and other organolithium compounds is estimated at 2000 to 3000 tonnes.[2]
Although butyllithium is colorless, n-butyllithium is usually encountered as a pale yellow solution in alkanes. Such solutions are stable indefinitely if properly stored,[3]but in practice, they degrade upon aging. Fine white precipitate (lithium hydroxide) is deposited and the color changes to orange.[3][4]
| |||
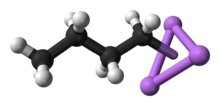 Close-up of the delocalized bonds between butyl and lithium | |||
| Names | |
|---|---|
| IUPAC name butyllithium, tetra-μ3-butyl-tetralithium | |
| Other names NBL, BuLi, 1-lithiobutane |

No comments:
Post a Comment