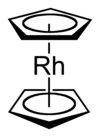
dilithioferrocene
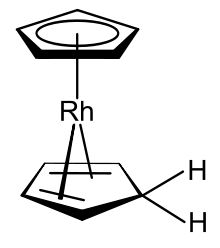
Rhodocene is a chemical compound with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds.[2] The [Rh(C5H5)2] radical is found above 150 °C or when trapped by cooling to liquid nitrogen temperatures (−196 °C). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.[1][3][4]
The history of organometallic chemistry includes the 19th-century discoveries of Zeise's salt[5][6][7] and nickel tetracarbonyl.[2] These compounds posed a challenge to chemists as the compounds did not fit with existing chemical bonding models. A further challenge arose with the discovery of ferrocene,[8] the iron analogue of rhodocene and the first of the class of compounds now known as metallocenes.[9]Ferrocene was found to be unusually chemically stable,[10] as were analogous chemical structures including rhodocenium, the unipositive cation of rhodocene[Note 1]and its cobalt and iridium counterparts.[11] The study of organometallic species including these ultimately led to the development of new bonding models that explained their formation and stability.[12][13] Work on sandwich compounds, including the rhodocenium-rhodocene system, earned Geoffrey Wilkinson and Ernst Otto Fischer the 1973 Nobel Prize for Chemistry.[14][15]
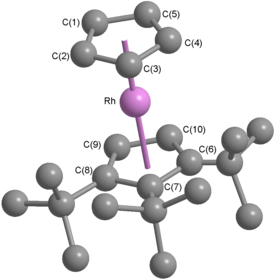
Owing to their stability and relative ease of preparation, rhodocenium salts are the usual starting material for preparing rhodocene and substituted rhodocenes, all of which are unstable. The original synthesis used a cyclopentadienyl anion and tris(acetylacetonato)rhodium(III);[11] numerous other approaches have since been reported, including gas-phase redox transmetalation[16] and using half-sandwichprecursors.[17] Octaphenylrhodocene (a derivative with eight phenyl groups attached) was the first substituted rhodocene to be isolated at room temperature, though it decomposes rapidly in air. X-ray crystallography confirmed that octaphenylrhodocene has a sandwich structure with a staggered conformation.[18] Unlike cobaltocene, which has become a useful one-electron reducing agent in research,[19] no rhodocene derivative yet discovered is stable enough for such applications.
Biomedical researchers have examined the applications of rhodium compounds and their derivatives in medicine[20] and reported one potential application for a rhodocene derivative as a radiopharmaceutical to treat small cancers.[21][22]Rhodocene derivatives are used to synthesise linked metallocenes so that metal–metal interactions can be studied;[23] potential applications of these derivatives include molecular electronics and research into the mechanisms of catalysis.[24] The value of rhodocenes tends to be in the insights they provide into the bonding and dynamics of novel chemical systems, rather than their applications.

| Names | |
|---|---|
| IUPAC name Rhodocene | |
| Other names bis(cyclopentadienyl)rhodium(II) dicyclopentadienylrhodium |





Rubidium is the chemical element with the symbol Rb and atomic number37. Rubidium is a very soft, silvery-white metal in the alkali metal group. Rubidium metal shares similarities to potassium metal and caesium metal in physical appearance, softness and conductivity.[6] Rubidium cannot be stored under atmospheric oxygen, as a highly exothermic reaction will ensue, sometimes even resulting in the metal catching fire.[7]
Rubidium is the first alkali metal in the group to have a density higher than water, so it sinks, unlike the metals above it in the group. Rubidium has a standard atomic weight of 85.4678. On Earth, natural rubidium comprises two isotopes: 72% is a stable isotope 85Rb, and 28% is slightly radioactive 87Rb, with a half-life of 48.8 billion years—more than three times as long as the estimated age of the universe.
https://en.wikipedia.org/wiki/Rubidium
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is an extraordinarily rare, silvery-white, hard, corrosion-resistant, and chemically inerttransition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isotope, 103Rh. Naturally occurring rhodium is usually found as a free metal, as an alloy with similar metals, and rarely as a chemical compound in minerals such as bowieite and rhodplumsite. It is one of the rarest and most valuable precious metals.
Rhodium is found in platinum or nickel ores together with the other members of the platinum group metals. It was discovered in 1803 by William Hyde Wollaston in one such ore, and named for the rose color of one of its chlorine compounds.
https://en.wikipedia.org/wiki/Rhodium

No comments:
Post a Comment