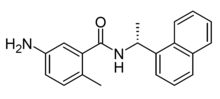
GRL-0617 is a drug which is one of the first compounds discovered that acts as a selective small-molecule inhibitor of the protease enzyme papain-like protease(PLpro) found in some pathogenic viruses, including the coronavirus SARS-CoV-2. It has been shown to inhibit viral replication in vitro.[1][2][3][4]
https://en.wikipedia.org/wiki/GRL-0617

Sulpiride has been studied for use as a hormonal contraceptive in women in whom conventional oral contraceptives are contraindicated and to potentiate progestogen-only contraceptives.[42][43] The contraceptive effects of sulpiride are due to its prolactin-releasing and antigonadotropic effects and the hyperprolactinemia–amenorrhea state that it induces.[42][43]
https://en.wikipedia.org/wiki/Sulpiride
3CLpro-1 is an antiviral drug related to rupintrivir which acts as a 3CL protease inhibitor and was originally developed for the treatment of human enterovirus 71. It is one of the most potent of a large series of compounds developed as inhibitors of the viral enzyme 3CL protease, with an in vitro IC50 of 200 nM. It also shows activity against coronavirus diseases such as SARS and MERS, and is under investigation as a potential treatment agent for the viral disease COVID-19.[1][2][3][4][5][6][7]

https://en.wikipedia.org/wiki/3CLpro-1
Papain belongs to a family of related proteins with a wide variety of activities, including endopeptidases, aminopeptidases, dipeptidyl peptidases and enzymes with both exo- and endopeptidase activity.[2] Members of the papain family are widespread, found in baculoviruses,[3] eubacteria, yeast, and practically all protozoa, plants and mammals.[2] The proteins are typically lysosomal or secreted, and proteolytic cleavage of the propeptide is required for enzyme activation, although bleomycin hydrolase is cytosolic in fungi and mammals.[4] Papain-like cysteine proteinases are essentially synthesised as inactive proenzymes (zymogens) with N-terminal propeptide regions. The activation process of these enzymes includes the removal of propeptide regions, which serve a variety of functions in vivo and in vitro. The pro-region is required for the proper folding of the newly synthesised enzyme, the inactivation of the peptidase domain and stabilisation of the enzyme against denaturing at neutral to alkaline pH conditions. Amino acid residues within the pro-region mediate their membrane association, and play a role in the transport of the proenzyme to lysosomes. Among the most notable features of propeptides is their ability to inhibit the activity of their cognate enzymes and that certain propeptides exhibit high selectivity for inhibition of the peptidases from which they originate.[5]
https://en.wikipedia.org/wiki/Papain
Baculoviridae is a family of viruses. Arthropods, lepidoptera, hymenoptera, and dipteraserve as natural hosts. There are 85 known species in this family, assigned to four genera.[1][2][3]
Baculoviruses are known to infect insects, with over 600 host species having been described. Immature (larval) forms of lepidopteran species (moths and butterflies) are the most common hosts, but these viruses have also been found infecting sawflies, and mosquitoes. Although baculoviruses are capable of entering mammalian cells in culture[4]they are not known to be capable of replication in mammalian or other vertebrate animal cells.
Starting in the 1940s they were used and studied widely as biopesticides in crop fields. Baculoviruses contain a circular double-stranded DNA (dsDNA) genome ranging from 80 to 180 kbp.
https://en.wikipedia.org/wiki/Baculoviridae



Chlorothiazide, sold under the brand name Diuril among others, is an organic compound used as a diuretic and as an antihypertensive.[1][2]
It is used both within the hospital setting or for personal use to manage excess fluid associated with congestive heart failure. Most often taken in pill form, it is usually taken orally once or twice a day. In the ICU setting, chlorothiazide is given to diurese a patient in addition to furosemide (Lasix). Working in a separate mechanism from furosemide and absorbed enterically as a reconstituted suspension administered through a nasogastric tube (NG tube), the two drugs potentiate one another.
It was patented in 1956 and approved for medical use in 1958.[3]

https://en.wikipedia.org/wiki/Chlorothiazide
Thiazide (/ˈθaɪəzaɪd/) refers to both a class of sulfur-containing organic molecules[1] and a class of diuretics based on the chemical structure of benzothiadiazine.[2] The thiazide drug class was discovered and developed at Merck and Co. in the 1950s.[3] The first approved drug of this class, chlorothiazide, was marketed under the trade name Diuril beginning in 1958.[3] In most countries, thiazides are the least expensive antihypertensive drugsavailable.[4]
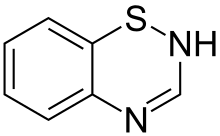
Thiazide organic molecules are bi-cyclic structures that contain adjacent sulfur and nitrogen atoms on one ring.[5] Confusion sometimes occurs because thiazide-like diureticssuch as indapamide are referred to as thiazides despite not having the thiazide chemical structure.[6] When used this way, "thiazide" refers to a drug which acts at the thiazide receptor.[7] The thiazide receptor is a sodium-chloride transporter that pulls NaCl from the lumen in the distal convoluted tubule. Thiazide diuretics inhibit this receptor, causing the body to release NaCl and water into the lumen, thereby increasing the amount of urine produced each day.[6] An example of a molecule that is chemically a thiazide but not used as a diuretic is methylchloroisothiazolinone, often found as an antimicrobial in cosmetics.[8]

https://en.wikipedia.org/wiki/Thiazide

https://en.wikipedia.org/wiki/Mesoridazine
Bromperidol (marketed as Bromidol, Bromodol) is a butyrophenone derivative. It is a potent and long-acting neuroleptic, used as an antipsychotic in the treatment of schizophrenia. It was discovered at Janssen Pharmaceutica in 1966.

https://en.wikipedia.org/wiki/Bromperidol
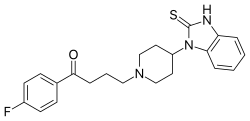
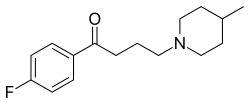
https://en.wikipedia.org/wiki/Melperone
https://en.wikipedia.org/wiki/Timiperone

FATULANT
https://en.wikipedia.org/wiki/Aripiprazole_lauroxil
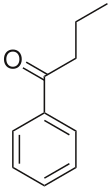
https://en.wikipedia.org/wiki/Butyrophenone
Industrial chemical insecticide CL1.
https://en.wikipedia.org/wiki/Category:Benzamides

Benzamide is a white solid with the chemical formula of C6H5C(O)NH2. It is the simplest amide derivative of benzoic acid. It is slightly soluble in water, and soluble in many organic solvents. A number of substituted benzamides are commercial drugs: sulpiride, remoxipride, amisulpride, tiapride, sultopride, veralipride, aminohippuric acid, cisapride, imatinib, and procainamide.
https://en.wikipedia.org/wiki/Benzamide
https://en.wikipedia.org/wiki/ATC_code_N05#N05AL_Benzamides

https://en.wikipedia.org/wiki/Teflurane
https://en.wikipedia.org/wiki/Category:Benzamides

https://en.wikipedia.org/wiki/IBNtxA
https://en.wikipedia.org/wiki/Category:Phenols
https://en.wikipedia.org/wiki/Category:Drugs_not_assigned_an_ATC_code
No comments:
Post a Comment