A diol is a chemical compound containing two hydroxyl groups (−OH groups).[1] An aliphatic diol is also called a glycol.[2] This pairing of functional groups is pervasive, and many subcategories have been identified.
The most common industrial diol is ethylene glycol. Examples of diols in which the hydroxyl functional groups are more widely separated include 1,4-butanediol HO−(CH2)4−OH and propylene-1,3-diol, or beta propylene glycol, HO−CH2−CH2−CH2−OH.
https://en.wikipedia.org/wiki/Diol
Propylene glycol (IUPAC name: propane-1,2-diol) is a viscous, colorless liquid, which is nearly odorless but possesses a faintly sweet taste. Its chemical formula is CH3CH(OH)CH2OH. Containing two alcohol groups, it is classed as a diol. It is miscible with a broad range of solvents, including water, acetone, and chloroform. In general, glycols are non-irritating and have very low volatility.[4]
It is produced on a large scale primarily for the production of polymers. In the European Union, it has E-number E1520 for food applications. For cosmetics and pharmacology, the number is E490. Propylene glycol is also present in propylene glycol alginate, which is known as E405. Propylene glycol is a compound which is GRAS (generally recognized as safe) by the US Food and Drug Administration under 21 CFR x184.1666, and is also approved by the FDA for certain uses as an indirect food additive. Propylene glycol is approved and used as a vehicle for topical, oral, and some intravenous pharmaceutical preparations in the U.S. and in Europe.

| Names | |
|---|---|
| Preferred IUPAC name Propane-1,2-diol | |
| Other names Propylene glycol α-Propylene glycol 1,2-Propanediol 1,2-Dihydroxypropane Methyl ethyl glycol Methylethylene glycol |
| Related compounds | |
|---|---|
Related glycols | Ethylene glycol, 1,3-propanediol |
Propylene glycol is also used in various edible items such as coffee-based drinks, liquid sweeteners, ice cream, whipped dairy products and soda.[9][10] Vaporizers used for delivery of pharmaceuticals or personal-care products often include propylene glycol among the ingredients.[4] In alcohol-based hand sanitizers, it is used as a humectant to prevent the skin from drying.[11] Propylene glycol is used as a solvent in many pharmaceuticals, including oral, injectable, and topical formulations. Many pharmaceutical drugs which are insoluble in water utilize propylene glycol as a solvent and carrier; benzodiazepine tablets are one example.[12]Propylene glycol is also used as a solvent and carrier for many pharmaceutical capsule preparations. Additionally, certain formulations of artificial tears use propylene glycol as an ingredient.[13]
The freezing point of water is depressed when mixed with propylene glycol. It is used as aircraft de-icing fluid.[4][14] Water-propylene glycol mixtures dyed pink to indicate the mixture is relatively nontoxic are sold under the name of RV or marine antifreeze. Propylene glycol is frequently used as a substitute for ethylene glycol in low toxicity, environmentally friendly automotive antifreeze. It is also used to winterize the plumbing systems in vacant structures.[15] The eutectic composition/temperature is 60:40 propylene glycol:water/-60 °C.[16][17] The −50 °F/−45 °C commercial product is, however, water rich; a typical formulation is 40:60.[18]
Along with vegetable glycerin as the main ingredient (<1–92%) in e-liquid used in electronic cigarettes, where it is aerosolized to resemble smoke. It serves as both the carrier for substances like nicotine and cannabinoids, as well as for creating a vapor which resembles smoke.[19]
Propylene glycol is expected to degrade rapidly in water from biological processes, but is not expected to be significantly influenced by hydrolysis, oxidation, volatilization, bioconcentration, or adsorption to sediment.[70] Propylene glycol is readily biodegradable under aerobic conditions in freshwater, in seawater and in soil. Therefore, propylene glycol is considered as not persistent in the environment.
https://en.wikipedia.org/wiki/Propylene_glycol
- Household chemicals
- Alcohol solvents
- Cosmetics chemicals
- Food additives
- Excipients
- Alkanediols
- Commodity chemicals
- Vicinal diols
- E-number additives
Isophthalic acid is an organic compound with the formula C6H4(CO2H)2. This colorless solid is an isomer of phthalic acid and terephthalic acid. The main industrial uses of purified isophthalic acid (PIA) are for the production of polyethylene terephthalate (PET) resin and for the production of unsaturated polyester resin (UPR) and other types of coating resins.
Isophthalic acid is one of three isomers of benzenedicarboxylic acid, the others being phthalic acid and terephthalic acid.

https://en.wikipedia.org/wiki/Isophthalic_acid
Maleic anhydride is an organic compound with the formula C2H2(CO)2O. It is the acid anhydride of maleic acid. It is a colorless or white solid with an acrid odor. It is produced industrially on a large scale for applications in coatings and polymers.[5]

https://en.wikipedia.org/wiki/Maleic_anhydride
Methylglyoxal (MGO) is the organic compound with the formula CH3C(O)CHO. It is a reduced derivative of pyruvic acid. It is a reactive compound that is implicated in the biology of diabetes. Methylglyoxal is produced industrially by degradation of carbohydrates using overexpressed methylglyoxal synthase.[1]
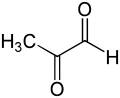
https://en.wikipedia.org/wiki/Methylglyoxal
Pivalonitrile is a nitrile with the semi-structural formula (CH3)3CCN, abbreviated t-BuCN. This aliphatic organic compound is a clear, colourless liquid that is used as a solvent and as a labile ligand in coordination chemistry. Pivalonitrile is isomeric with tert-butyl isocyanide but the two compounds do not exist in chemical equilibrium, unlike its silicon analog trimethylsilyl cyanide. [2]

https://en.wikipedia.org/wiki/Pivalonitrile
Aminoacetonitrile is the organic compound with the formula NCCH2NH2. The compound is a colorless liquid. It is unstable at room temperature, owing to the incompatibility of the amine nucleophile and the nitrile electrophile. For this reason it is usually encountered as the chloride and bisulfate salts of the ammoniumderivative, i.e., [NCCH2NH3]+Cl− and [NCCH2NH3]+HSO4−.[3]
![]()

https://en.wikipedia.org/wiki/Aminoacetonitrile
Cyanogen is the chemical compound with the formula (CN)2. It is a colorless, toxic gas with a pungent odor. The molecule is a pseudohalogen. Cyanogen molecules consist of two CN groups – analogous to diatomic halogen molecules, such as Cl2, but far less oxidizing. The two cyano groups are bonded together at their carbonatoms: N≡C−C≡N, although other isomers have been detected.[6] The name is also used for the CN radical,[7] and hence is used for compounds such as cyanogen bromide (NCBr).[8]
Cyanogen is the anhydride of oxamide:
- H2NC(O)C(O)NH2 → NCCN + 2 H2O
although oxamide is manufactured from cyanogen by hydrolysis:[9]
- NCCN + 2 H2O → H2NC(O)C(O)NH2
![]()
| Names | |
|---|---|
| Preferred IUPAC name Oxalonitrile[4] | |
| Systematic IUPAC name Ethanedinitrile[4] | |
| Other names |
https://en.wikipedia.org/wiki/Cyanogen
Oxamide is the organic compound with the formula (CONH2)2. This white crystallinesolid is soluble in ethanol, slightly soluble in water and insoluble in diethyl ether. Oxamide is the diamide derived from oxalic acid.

| Names | |
|---|---|
| Preferred IUPAC name Oxamide[1] | |
| Systematic IUPAC name Ethanediamide | |
| Other names Oxalamide Oxamimidic acid Diaminoglyoxal Oxalic acid diamide 1-Carbamoyl-formimidic acid |
https://en.wikipedia.org/wiki/Oxamide
Oxanamide (Quiactin) is an anxiolytic and muscle relaxant which can produce sedative and hypnotic effects in sufficiently high doses.[1] An uncontrolled trial on patients treated in a clinical gynecology practice published in 1959 found that oxanamide was efficacious in the treatment of anxiety resulting from premenstrual syndrome, menopause, and various other causes, with minimal sedation or other side effects.[2]
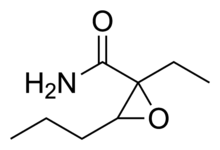
https://en.wikipedia.org/wiki/Oxanamide
Category:Carboxamides
From Wikipedia, the free encyclopedia
Jump to navigationJump to search
The main article for this category is Carboxamide.
Wikimedia Commons has media related to Carboxamides.
Subcategories
This category has the following 19 subcategories, out of 19 total.
A
Acetamides (2 C, 172 P)
Acrylamides (18 P)
Acylguanidines (7 P)
Anilides (3 C, 113 P)
B
Benzamides (3 C, 181 P)
Benzofuran-2-carboxamides (4 P)
Butyramides (9 P)
C
Carbamates (11 C, 186 P)
F
Fatty acid amides (31 P)
Formamides (31 P)
I
Indazolecarboxamides (34 P)
Indolecarboxamides (21 P)
Isonicotinamides (8 P)
L
Lactams (22 C, 327 P)
Lysergamides (1 C, 43 P)
N
Nicotinamides (17 P)
P
Peptides (9 C, 276 P)
Propionamides (63 P)
Pyrazolecarboxamides (8 P)
Pages in category "Carboxamides"
The following 200 pages are in this category, out of approximately 350 total. This list may not reflect recent changes (learn more).
(previous page) (next page)
Amide
A
A-836,339
Abitesartan
Acemetacin
Acotiamide
Acrydite
Acrylamide
N-Acyl homoserine lactone
Adatanserin
ADB-CHMINACA
Adelmidrol
Adipamide
Advantame
Afatinib
Ageliferin
Alacepril
Alfuzosin
Alpidem
Alvimopan
Ambenonium chloride
ANA-12
Andarine
Annimycin
Apimostinel
Arotinolol
Arterolane
Asoxime chloride
Asparagine
AT-076
Atevirdine
Avanafil
B
Barusiban
Beclamide
Befiradol
Benznidazole
Bevenopran
Biocytin
Biotin PEG2 amine
Bistramide A
BMS-470539
Bortezomib
BP-897
Brivaracetam
Brostallicin
Bucladesine
Bunaftine
Bunazosin
Bupicomide
C
Candoxatril
Capsinolol
Captopril
8-Carboxamidocyclazocine
5-Carboxamidotryptamine
Carboxyamidotriazole
Carpipramine
Cefacetrile
Cefmetazole
Cellocidin
Ceronapril
Cerulenin
CGS-21680
Chavicine
CI-988
Cinchocaine
Cinepazet
Cinepazide
CJ-033466
Cl-4AS-1
Clindamycin
Clocapramine
Clofibride
Clovamide
Coelenteramide
Coenzyme B
Colibactin
Complanine
Coronatine
CP-532,903
CUMYL-CBMICA
CX-516
D
Dabigatran
Dacarbazine
Dacemazine
Dagrocorat
Dapagliflozin/saxagliptin
Darifenacin
Darolutamide
Deferoxamine
Delavirdine
Demeclocycline
Dextromoramide
Dicrotophos
Dihydrolipoamide
Dimethenamid
Dimethoate
Disopyramide
Doxazosin
Droxinavir
Dutasteride
Dysidenin
E
Ebalzotan
Eclanamine
Eliglustat
Elinzanetant
Embutramide
Encenicline
Endralazine
Entacapone
Erdosteine
Esaxerenone
Etiracetam
Evenamide
F
Fagaramide
Faldaprevir
Farampator
Fasoracetam
Favipiravir
Fenoverine
FG-7142
Finasteride
Finerenone
Fipexide
Flomoxef
Fluacizine
Fluxapyroxad
Fosdagrocorat
Fosinopril
Frovatriptan
Furylfuramide
G
GelGreen
GelRed
Gemopatrilat
Glecaprevir
Glicaramide
Glisoxepide
Glutamine
Glycidamide
Glycinamide
GNF6702
GSK-189254
Guineesine
H
HIOC
Hydracarbazine
I
Ibodutant
ICI-190,622
ICI-164384
Idrocilamide
Iferanserin
Ilepcimide
Imidafenacin
Imidapril
Imidazenil
Indecainide
Indinavir
Indolepropionamide
Indometacin
Isobutyramide
Isopropamide
Isovaleramide
ISRIB
J
JDTic
JNJ-7777120
JTE-907
JZ-IV-10
K
K-Ras(G12C) inhibitor 6
Ketodarolutamide
L
L-765,314
Lactamide
Lapaquistat
Lapisteride
Lasinavir
Lazabemide
Lemborexant
Lenvatinib
Levomilnacipran
Levomoramide
Lipoamide
Lisinopril
LM22A-4
Lodoxamide
Lornoxicam
Lucitanib
Lufuradom
Luminespib
Lusutrombopag
LY-456219
LY-456220
M
Maraviroc
Mazapertine
Mefway (18F)
Meloxicam
Meropenem
Methylamide
Metopimazine
Milnacipran
Mirfentanil
Mitiglinide
Mitozolomide
MK-0773
Monatepil
JZ-IV-10 is a piperidine derivative related to cocaine which acts as a highly potent serotonin–norepinephrine–dopamine reuptake inhibitor (also called SNDRI, or triple reuptake inhibitor).[1] The eugeroic modafinil was used as a lead to fuel this compound's discovery.[2][3]
See also
[edit]
- 1-Methyl-3-propyl-4-(p-chlorophenyl)piperidine
- N,O-Dimethyl-4-(2-naphthyl)piperidine-3-carboxylate
- Nocaine
4[FeII
(CN)
6]
3. Turnbull's blue is chemically identical, but is made from different reagents, and its slightly different color stems from different impurities.
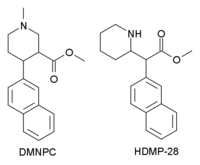
N,O-Dimethyl-4β-(2-naphthyl)piperidine-3β-carboxylate (DMNPC) is a piperidinebased stimulant drug which is synthesised from arecoline. It is similar to nocaine in chemical structure, and has two and a half times more activity than cocaine as a dopamine reuptake inhibitor. However it is also a potent serotonin reuptake inhibitor, with similar affinity to fluoxetine.[1]
1-Methyl-3-propyl-4-(p-chlorophenyl)piperidine is a drug developed by a team led by Alan Kozikowski, which acts as a potent dopamine reuptake inhibitor, and was developed as a potential therapeutic agent for the treatment of cocaine addiction.[1]As with related compounds such as nocaine, it is a structurally simplified derivative of related phenyltropane compounds.[2] Its activity at the serotonin and noradrenalinetransporters has not been published, though most related 4-phenylpiperidine derivatives are relatively selective for inhibiting dopamine reuptake over the other monoamine neurotransmitters. While several of its isomers are active, the (3S,4S)-enantiomer is by far the most potent.[3][4] The rearranged structural isomer 2-[1-(4-chlorophenyl)butyl]piperidine is also a potent inhibitor of dopamine reuptake.[5]
See also[edit]

Pages in category "4-Phenylpiperidines"
The following 77 pages are in this category, out of 77 total. This list may not reflect recent changes (learn more).
0–9
D
M
P
W
4-Phenylpiperidine is a chemical compound. It features a benzene ring bound to a piperidine ring.
4-Phenylpiperidine is the base structure for a variety of opioids, such as pethidine(meperidine), ketobemidone, alvimopan, loperamide, and diphenoxylate.
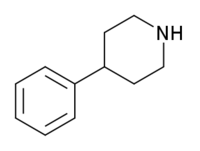
https://en.wikipedia.org/wiki/4-Phenylpiperidine
- Stimulants
- Carboxamides
- 4-Phenylpiperidines
- Chloroarenes
- Serotonin-norepinephrine-dopamine reuptake inhibitors
- Nervous system drug stubs
Stimulants
showvte
Monoamine reuptake inhibitors
https://en.wikipedia.org/wiki/Template:Monoamine_neurotoxins
https://en.wikipedia.org/wiki/JZ-IV-10
https://en.wikipedia.org/wiki/Template:Monoamine_metabolism_modulators
Pipradrol (Meratran) is a mild central nervous system stimulant (norepinephrine-dopamine reuptake inhibitor) that is no longer widely used in most countries due to concerns about its abuse potential. Pipradrol is still used in some European countries, and even in the United States, albeit rarely.
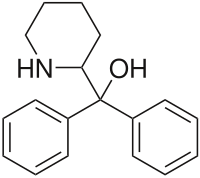
Pipradrol was developed in the 1940s,[1] and found use initially for treating obesity.[2]It was subsequently used for the treatment of a variety of other conditions such as narcolepsy, ADHD, and most particularly for counteracting the symptoms of senile dementia, this being the only application for which it is still used medically.[medical citation needed]
Common side effects include insomnia, anorexia, tachycardia, and anxiety. Rarer side effects include dry mouth, tremor, hypertension, euphoria, depression, etc..
https://en.wikipedia.org/wiki/Pipradrol
Dopexamine is a synthetic analogue of dopamine that is administered intravenously in hospitals to reduce exacerbations of heart failure and to treat heart failure following cardiac surgery. It is not used often, as more established drugs like epinephrine, dopamine, dobutamine, norepinephrine, and levosimendan work as well. It works by stimulating beta-2 adrenergic receptors and peripheral dopamine receptor D1 and dopamine receptor D2. It also inhibits the neuronal re-uptake of norepinephrine.
The most common adverse effects include fast heart beats and nausea.
It was discovered by scientists at Fisons, which licensed it to Ipsen in 1993, and Ipsen in turn licensed it to Élan in 1999. Ipsen licensed rights in North America and Japan to Circassia in 2008; the drug had never been approved in those countries. Dopexamine went off-patent in 2010.
https://en.wikipedia.org/wiki/Dopexamine
An inotrope[help 1] is an agent that alters the force or energy of muscular contractions. Negatively inotropic agents weaken the force of muscular contractions. Positively inotropic agents increase the strength of muscular contraction.
The term inotropic state is most commonly used in reference to various drugs that affect the strength of contraction of heartmuscle (myocardial contractility). However, it can also refer to pathological conditions. For example, enlarged heart muscle (ventricular hypertrophy) can increase inotropic state, whereas dead heart muscle (myocardial infarction) can decrease it.
https://en.wikipedia.org/wiki/Inotrope
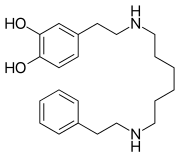
Glycerol (/ˈɡlɪsərɒl/;[6] also called glycerine in British English or glycerin in American English) is a simple polyol compound. It is a colorless, odorless, viscousliquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipidsknown as glycerides. Due to having antimicrobial and antiviral properties it is widely used in FDA approved wound and burn treatments. It can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Owing to the presence of three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature.[7]
https://en.wikipedia.org/wiki/Glycerol
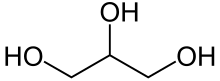
Propylene oxide is an organic compound with the molecular formula CH3CHCH2O. This colourless volatile liquid with an odour resembling ether, is produced on a large scale industrially. Its major application is its use for the production of polyether polyols for use in making polyurethane plastics. It is a chiral epoxide, although it is commonly used as a racemic mixture.
This compound is sometimes called 1,2-propylene oxide to distinguish it from its isomer 1,3-propylene oxide, better known as oxetane.
| Names | |
|---|---|
| Preferred IUPAC name (2R)-2-Methyloxirane (2S)-2-Methyloxirane | |
| Other names Propylene oxide Epoxypropane Propylene epoxide 1,2-Propylene oxide Methyl oxirane 1,2-Epoxypropane Propene oxide Methyl ethylene oxide Methylethylene oxide |
https://en.wikipedia.org/wiki/Propylene_oxide
Dipropylene glycol is a mixture of three isomeric chemical compounds, 4-oxa-2,6-heptandiol, 2-(2-hydroxy-propoxy)-propan-1-ol, and 2-(2-hydroxy-1-methyl-ethoxy)-propan-1-ol. It is a colorless, nearly odorless liquid with a high boiling point and low toxicity.[2][3]
https://en.wikipedia.org/wiki/Dipropylene_glycol
Polypropylene glycol or polypropylene oxide is the polymer of propylene glycol. Chemically it is a polyether, and, more generally speaking, it's a polyalkylene glycol (PAG) H S Code 3907.2000. The term polypropylene glycol or PPG is reserved for low to medium range molar mass polymer when the nature of the end-group, which is usually a hydroxyl group, still matters. The term "oxide" is used for high molar mass polymer when end-groups no longer affect polymer properties. Between 60 and 70% of propylene oxide is converted to polyether polyols by the process called alkoxylation.[1]
https://en.wikipedia.org/wiki/Polypropylene_glycol
Dr.Robert Bettey
Dr. Bettey, a native of Orange County, majored in mechanical engineering at Cal State Fullerton before receiving his Doctor of Veterinary Medicine Degree from UC Davis. He founded Equine Medical Associates after graduating and has been practicing in Orange County for over 25 years. His primary area of interest is lameness including advanced therapies for tendon injuries. He is one of only a few veterinarians in the country to have completed a post graduate Fellowship in equine ultrasound at the UC Davis School of Veterinary Medicine. Outside of work he enjoys family time, volleyball, and classic cars.

| Poison Ivy | |
|---|---|
 Variant cover of Batman vol. 3, #26 (Sept. 2017) Art by Joshua Middleton |
Phosphorus Rex is a fiery member of the Circus of Strange.[1] After Mister Toad was apprehended by the Gotham City Police Department, Phosphorus Rex joined the Circus of Strange members Big Top, Siam, and a group of Professor Pyg's Dollotrons in attacking the Gotham City Police Department which resulted in four police officers getting killed and six others ending up injured. Phosphorus Rex and the rest of the Circus of Strange were defeated by Batman and Robin. Batman later interrogated Phosphorus Rex into giving him the identity of the Circus of Strange's leader. What Phosphorus Rex told Batman led him to Professor Pyg.[2]
In The New 52, Phosphorus Rex and Professor Pyg are first seen as inmates of Arkham Asylum. At the time when Batman and Nightwing arrived at Arkham Asylum to capture a corrupt guard who ended up releasing the inmates, Phosphorus Rex is among those who take part in the break-out attempt only to be thwarted by Batman and Nightwing.[3]
https://en.wikipedia.org/wiki/Phosphorus_Rex
| Doctor Phosphorus | |
|---|---|
 Doctor Phosphorus Art by Marcos Marz |
https://en.wikipedia.org/wiki/Doctor_Phosphorus
The Dollmaker is the name of several fictional supervillains appearing in American comic books published by DC Comics.
Barton Mathis appeared in the second season of the Arrowverse series Arrowplayed by actor Michael Eklund and in the first season of Gotham played by Colm Feore (renamed as Dr. Francis Dulmacher).
https://en.wikipedia.org/wiki/Dollmaker_(character)
Recurring enemies
Amygdala Anarky Black Spider Blockbuster Catman Cavalier Clock King Cluemaster Composite Superman Copperhead Cornelius Stirk Crazy Quilt Crime Doctor David Cain Deacon Blackfire Doctor Death Doctor Double X Doctor Phosphorus Dollmaker Electrocutioner Enigma Firebug Flamingo Gearhead Great White Shark Humpty Dumpty Jane Doe Joker's Daughter Key KGBeast King Snake Kite Man Kobra Lex Luthor Maxie Zeus Magpie Mirror Man Night-Slayer Nocturna Nora Fries Nyssa Raatko Onomatopoeia Orca Outsider Owlman Phosphorus Rex Planet Master Polka-Dot Man Professor Milo Prometheus Rag Doll Ratcatcher Reaper Sensei Signalman Simon Hurt Simon Stagg Snowman Spellbinder Swagman Talia al Ghul Tally Man Ten-Eyed Man Tiger Shark Tweedledum and Tweedledee Wrath Zebra-Man
Batman
Earth-Two Tlano Owlman The Batman Who Laughs Batzarro Thomas Wayne (Flashpoint version)
Dr. Death is a mad scientist and supervillain appearing in publications by DC Comics. The character was created by Gardner Fox and Bob Kane as an enemy of the superhero Batman, and first appeared in Detective Comics #29 (July 1939).[1] He is notable as the first traditional supervillain to be encountered by the Batman, as well as his first recurring foe.[2]
https://en.wikipedia.org/wiki/Doctor_Death_(character)
Nocturna (/nɒkˈtɜːrnə/) is a fictional supervillainess character appearing in comic books published by DC Comics, created by Doug Moench and Gene Colan. The storyline involving her began in Detective Comics #529 (August 1983), and her first appearance was in Batman #363 (September 1983).[1]
The Pre-Crisis incarnation of Nocturna appeared in the first season of the live-action Arrowverse series Batwoman, portrayed by Kayla Ewell.
https://en.wikipedia.org/wiki/Nocturna_(DC_Comics)
Mirror Man is the name of three different characters appearing in comic books published by DC Comics.
The Floyd Ventris version of Mirror Man has genius-level intellect and uses devices that are themed with mirrors.
The New Rogues version of Mirror Man uses Mirror Master's special mirrors in battle.
https://en.wikipedia.org/wiki/Mirror_Man_(character)
Mirror Master is the name of several fictional supervillains appearing in comic books published by DC Comics. He is a recurring foe of the Flash with considerable technical expertise and skills involving the use of mirrors.[1] Three individuals have donned the guise of Mirror Master (with a couple being members of the Rogues at different times). In 2009, Mirror Master was ranked as IGN's 79th Greatest Comic Book Villain of All Time.[2]
Both incarnations of Mirror Master have made several appearances in DC-related media, with Sam Scudder being portrayed in live-action by David Cassidyin the 1990 The Flash series and by Grey Damon in the 2014 The Flash series, while Efrat Dor portrayed a gender-swapped version of Evan McCulloch, renamed Eva McCulloch / Mirror Monarch, and reimagined as a CEO of her own company in the 2014 series.
https://en.wikipedia.org/wiki/Mirror_Master
The Creeper (Jack Ryder) is a superhero created by Steve Ditko and Don Segall for DC Comics. He is portrayed as a journalist and talk show host, usually living in Gotham City, who gains the ability to transform into the superhuman the Creeper (and vice versa) thanks to experimental science developed by Dr. Yatz. First appearing in Showcase #73 (March 1968), his origin was revised in Secret Origins (vol. 2) #18 in 1987, then partially revised again in The Creeper #1-4 in 1997, then completely reimagined in the six-issue miniseries The Creeper (vol. 2), published in 2006-2007.
https://en.wikipedia.org/wiki/Creeper_(DC_Comics)
Samuel Emerson "Slam" Bradley is a fictional character that has appeared in various comic book series published by DC Comics. He is a private detectivewho exists in DC's main shared universe. The character concept was envisioned by DC Comics founder Malcolm Wheeler-Nicholson. The character was created by collaboration of Jerry Siegel and Joe Shuster, who both later became more well known as the co-creators of Superman. As one of the first ever DC characters, the character first appears in the Golden Age of Comic Books in the anthology title Detective Comics, being introduced in the first issue. He later commonly was associated with Batman and other spinoff Batman characters when revived.
Slam Bradley was portrayed in live-action by Kurt Szarka in the first season of the Arrowverse series Batwoman.[1]
| Slam Bradley | |
|---|---|
 Interior artwork from Catwoman Secret Files & Origins vol. 1, 1 (September, 2002 DC Comics). Art by Michael Lark. |
https://en.wikipedia.org/wiki/Slam_Bradley
Man-Bat (Dr. Robert Kirkland "Kirk" Langstrom) is a fictional character appearing in comic books published by DC Comics. Introduced in Detective Comics #400 (June 1970) as an enemy of the superhero Batman,[1] the character belongs to the collective of adversaries that make up his rogues gallery. Originally portrayed as a supervillain, later incarnations show Man-Bat as a sympathetic villain or antihero.
Man-Bat originated as an anthropomorphic, feral, bat-like creature, lacking sentience and acting purely on instinct; the result of Langstrom testing a serum that could supposedly give humans a bat's acute sonar sense on himself. Batman managed to reverse the effects, but Langstrom would return as Man-Bat time and time again, albeit not necessarily as a villain, as Langstrom would sometimes retain enough sanity to use his powers for good. Several other characters have since appeared as similar Man-Bat creatures, including Langstrom's wife Francine and father Aaron.
Since his debut at the end of the Silver Age of Comic Books, Man-Bat has been featured in various media adaptations, including television series and video games. In 2017, Man-Bat was ranked as IGN's 16th best Batman villain.[2]
| Man-Bat | |
|---|---|
 The Man-Bat from Who's Who in the DC Universe#12, art by Michael Golden |
https://en.wikipedia.org/wiki/Man-Bat
Lady Shiva (real name Sandra Woosan, Sandra San or more recently Sandra Wu-San) is a fictional character appearing in comic books published by DC Comics. The character was co-created by Dennis O'Neil and Ric Estrada, and first appeared in Richard Dragon, Kung Fu Fighter #5.[3] Over time, she has become more closely associated with Batman and related characters, both as an enemy and an ally. She is a martial arts grandmaster and one of the most skilled combatants in the DC Universe. She is an assassin-for-hire who specializes in killing her targets with her bare hands, and is the mother of Cassandra Cain, a.k.a. Batgirl and Orphan.
| Lady Shiva | |
|---|---|
 Lady Shiva, as she appears on the cover of Birds of Prey vol. 2 #6 (Nov. 2010). Art by Alina Urusov. |
https://en.wikipedia.org/wiki/Lady_Shiva
Doctor Double X (originally called Doctor X and Double X) is a fictional character in the DC Comics universe. The character is a supervillain who has fought Batman and Robin several times in Gotham City.[1]
https://en.wikipedia.org/wiki/Doctor_Double_X
The Crime Doctor is the name of two fictional supervillains that appears in American comic books published by DC Comics. The Crime Doctor is an underworld medical expert who caters exclusively to criminals, originally an enemy of Batman.[1]
https://en.wikipedia.org/wiki/Crime_Doctor_(comics)
Gearhead is an alias used by two fictional characters, both supervillains appearing in American comic books published by DC Comics.
The unidentified Gearhead first appeared in Steel #14.
The Nathan Finch version of Gearhead first appeared in Detective Comics #712 and was created by Chuck Dixon, Graham Nolan, and Bob McLeod.
https://en.wikipedia.org/wiki/Gearhead_(DC_Comics)
The Clock King is the name of three fictional supervillains appearing in American comic books published by DC Comics. The first Clock King was a villain and enemy of Green Arrow, and debuted in World's Finest Comics #111 (August 1960), and was created by France Herron and Lee Elias.[1]
The Clock King made his first live-action appearance in the 1960s Batman TV series, played by Walter Slezak. The character was later played by Robert Knepper, appearing in episodes from the Arrow's second season and The Flashset in the Arrowverse.
| Clock King | |
|---|---|
 The Tem version of Clock King. |
https://en.wikipedia.org/wiki/Clock_King
Green Arrow is a fictional superhero who appears in comic books published by DC Comics. Created by Mort Weisinger and designed by George Papp, he first appeared in More Fun Comics #73 in November 1941. His real name is Oliver Jonas Queen, a wealthy businessman and owner of Queen Industries who is also a well-known celebrity in Star City. He uses this position to hide the fact that he is the Arrow.[1] Sometimes shown dressed like the character Robin Hood, Green Arrow is an archer who uses his skills to fight crime in his home cities of Star City and Seattle, as well as alongside his fellow superheroes as a member of the Justice League. Though much less frequently used in modern stories, he also deploys a range of trick arrows (in contemporary times, they are referred as "specialty arrows"[2]) with various special functions, such as glue, explosive-tipped, grappling hook, flash grenade, tear gas and even kryptonite arrows for use in a range of special situations. At the time of his debut, Green Arrow functioned in many ways as an archery-themed analogue of the very popular Batman character, but writers at DC subsequently developed him into a voice of left-wing politics very much distinct in character from Batman.
Green Lantern, in a ground-breaking, socially conscious comic book series.[3]
| Partnerships | Black Canary Speedy (various) Connor Hawke Green Lantern (Hal Jordan) |
|---|
https://en.wikipedia.org/wiki/Green_Arrow
He is later recruited by Alexander Luthor, Jr.'s Secret Society of Super Villains as part of an army that is sent to conquer the city of Metropolis in issue #7 of the 2006 miniseries Infinite Crisis. A superhero army, backed up by the National Guard, successfully opposes the Society. Onomatopoeia is seen in a brawl with the Odd Man, a costumed, un-powered vigilante.[citation needed]
https://en.wikipedia.org/wiki/Onomatopoeia_(comics)
Crazy-Quilt is the name of several characters in DC Comics.
https://en.wikipedia.org/wiki/Crazy_Quilt
Elongated Man (Randolph "Ralph" Dibny) is a fictional character, a superheroappearing in American comic books published by DC Comics. His first appearance was in The Flash #112 (February 25, 1960).[1]
https://en.wikipedia.org/wiki/Elongated_Man
Rag Doll (Peter Merkel Jr.) is a fictional character, a supervillain and anti-hero in the DC Comics Universe. He first appeared in Villains United #1 (July 2005), and was created by Gail Simone and Dale Eaglesham. He is a member of the Secret Six and the son of the original Rag Doll, Peter Merkel.
| Rag Doll | |
|---|---|
 |
https://en.wikipedia.org/wiki/Rag_Doll_(Peter_Merkel_Jr.)
Created by Apokoliptian scientist Himon using the mysterious Element X, they are generally thought to be sentient, miniaturized, portable supercomputers, although their true nature and origins are unknown.[1] They possess wondrous powers and abilities not understood even by their users, the gods of New Genesis. These range from teleportation (they can summon Boom Tubes) to energy manipulation, and Mother Boxes have even been seen healing the injured, including Darkseidhimself, after he was beaten by Doomsday. Metron stated that each Mother Box shares "a mystical rapport with nature". They provide their owner with unconditional love and self-destruct when their owner dies.
Mother Boxes have sacrificed themselves for causes they have believed in and are greatly respected by the people of New Genesis. In physical appearance they are most often in the shape of a small box, but they can also be much larger (as is the one carried by the Forever People), and do not always need to be in the shape of a box at all (Mr. Miracle had Mother Box circuitry woven into the hood of his costume). They usually communicate with a repetitive "ping!" which can be understood by their users.
| Mother Box | |
|---|---|
 |








No comments:
Post a Comment