Chlorodiphenylphosphine is an organophosphorus compound with the formula (C6H5)2PCl, abbreviated Ph2PCl. It is a colourless oily liquid with a pungent odor that is often described as being garlic-like and detectable even in the ppb range. It is useful reagent for introducing the Ph2P group into molecules, which includes many ligands.[2] Like other halophosphines, Ph2PCl is reactive with many nucleophilessuch as water and easily oxidized even by air.

| Names | |
|---|---|
| Preferred IUPAC name Diphenylphosphinous chloride[1] | |
| Other names chlorodiphenylphosphine p-chlorodiphenylphosphine diphenyl phosphine chloride diphenylchlorophosphine |
Chlorodiphenylphosphine is produced on a commercial scale from benzene and phosphorus trichloride (PCl3). Benzene reacts with phosphorus trichloride at extreme temperatures around 600 °C to give dichlorophenylphosphine (PhPCl2) and HCl. Redistribution of PhPCl2 in the gas phase at high temperatures results in chlorodiphenylphosphine.[2][3]
- 2 PhPCl2 → Ph2PCl + PCl3
Alternatively such compounds are prepared by redistribution reactions starting with triphenylphosphine and phosphorus trichloride.
- PCl3 + 2 PPh3 → 2 Ph2PCl
Chlorodiphenylphosphine hydrolyzes to give diphenylphosphine oxide. Reduction with sodium affords tetraphenyldiphosphine:
- 2 Ph2PCl + 2 Na → [Ph2P]2 + 2 NaCl
Chlorodiphenylphosphine, along with other chlorophosphines, is used in the synthesis of various phosphines. A typical route uses Grignard reagents:[3]
- Ph2PCl + MgRX → Ph2PR + MgClX
The phosphines produced from reactions with Ph2PCl are further developed and used as pesticides (such as EPN), stabilizers for plastics (Sandostab P-EPQ), various halogen compound catalysts, flame retardants (cyclic phosphinocarboxylic anhydride), as well as UV-hardening paint systems (used in dental materials) making Ph2PCl an important intermediate in the industrial world.[2][3]
https://en.wikipedia.org/wiki/Chlorodiphenylphosphine
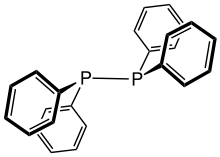
Tetraphenyldiphosphine is the organophosphorus compound with the formula [PPh2]2, where Ph = phenyl (C6H5). It is a white, air-sensitive solid that dissolves in nonpolar solvents. It is a centrosymmetric molecule with a P-P bond of 2.2592 Å.[1]
Tetraphenyldiphosphine is produced by reductive coupling of chlorodiphenylphosphine:
- 2 Ph2PCl + 2 Na → Ph2P-PPh2 + 2 NaCl
The compound is used as a source of the Ph2P− group.[2]
- Ph2P-PPh2 + 2 Na → + 2 NaPPh2
https://en.wikipedia.org/wiki/Tetraphenyldiphosphine
Diphenylphosphine oxide is an organophosphorus compound with the formula (C6H5)2P(O)H. It is a white solid that soluble in polar organic solvents. The compound is used in Buchwald-Hartwig coupling reactions to introduce a diphenylphosphino substituent.[1] Analogous to the behavior of phosphorous acid, diphenylphosphine oxide exists in equilibrium with a minor tautomer hydroxydiphenylphosphine (CAS#24630-80-6) (C6H5)2POH.

| Names | |
|---|---|
| Preferred IUPAC name Diphenyl-λ5-phosphanone |
Diphenylphosphine oxide can be prepared by the reaction of phosphonic esters, such as diethylphosphite, with Grignard reagents. Alternatively, it may be prepared by the partial hydrolysis of chlorodiphenylphosphine[1] or diphenylphosphine.[2]
Reactions[edit]
Organophosphinous acids are deoxygenated with DIBAH. The resulting secondary phosphines are precursors to phosphine ligands.[3]
https://en.wikipedia.org/wiki/Diphenylphosphine_oxide
Dichlorophenylphosphine is an organophosphorus compound with the formulaC6H5PCl2. This colourless viscous liquid is commonly used in the synthesis of phosphine ligands.
Dichlorophenylphosphine is commercially available. It may be prepared by an electrophilic substitution of benzene by phosphorus trichloride, catalyzed by aluminium chloride.[1] The compound is an intermediate for the synthesis of other chemicals for instance dimethylphenylphosphine: DOI:
- C6H5PCl2 + 2 CH3MgI → C6H5P(CH3)2 + 2 MgICl
In the McCormack reaction dichlorophenylphosphine adds dienes to give the chlorophospholenium ring.[2]

| Names | |
|---|---|
| Preferred IUPAC name Phenylphosphonous dichloride | |
| Other names Dichlorophenylphosphane Phenylphosphorus dichloride |
https://en.wikipedia.org/wiki/Dichlorophenylphosphine
1,4-Dioxane (/daɪˈɒkseɪn/) is a heterocyclic organic compound, classified as an ether. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. The compound is often called simply dioxane because the other dioxane isomers (1,2-and 1,3-) are rarely encountered.
Dioxane is used as a solvent for a variety of practical applications as well as in the laboratory, and also as a stabilizer for the transport of chlorinated hydrocarbons in aluminum containers.[3]

| Names | |
|---|---|
| Preferred IUPAC name 1,4-Dioxane | |
| Systematic IUPAC name 1,4-Dioxacyclohexane | |
| Other names [1,4]Dioxane p-Dioxane [6]-crown-2 Diethylene dioxide Diethylene ether Dioxan |
https://en.wikipedia.org/wiki/1,4-Dioxane
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometalliccompounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

https://en.wikipedia.org/wiki/Triphenylphosphine#Conversion_to_PPh2_derivatives

No comments:
Post a Comment