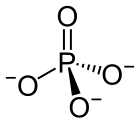
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid H
3PO
4.
The phosphate or orthophosphate ion [PO
4]3−
is derived from phosphoric acid by the removal of three protons H+
. Removal of one or two protons gives the dihydrogen phosphate ion [H
2PO
4]−
and the hydrogen phosphate ion [HPO
4]2−
ion, respectively. These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate.
https://en.wikipedia.org/wiki/Phosphate
This article is about the orthophosphate ion. For the organophosphorus derivatives, see Organophosphate. For other phosphates, see phosphoric acids and phosphates.
Not to be confused with phosphate soda or phosphonate.

No comments:
Post a Comment