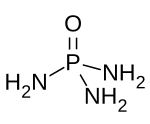
Phosphoramide is a chemical compound with the molecular formula O=P(NH2)3. It is a derivative of phosphoric acid in which each of the hydroxyl groups have been replaced with an amino group. Phosphoramide arises from the reaction of phosphoryl chloride with ammonia. It is a white solid that is soluble in polar solvents. In moist air, it hydrolyzes to an ammonium salt:
- 2 H2O + OP(NH2)3 → NH4+[HPO3(NH2)] + NH3
It reacts with sodium hydroxide with loss of ammonia:[1]
- NaOH + OP(NH2)3 → NaO2P(NH2)2 + NH3
The related thiophosphoryl compound P(=S)(NH2)3 was made from the reaction of thiophosphoryl chloride with ammonia.
 | |
| Names | |
|---|---|
| IUPAC name Phosphoric triamide | |
| Other names Phosphoric amide; Diaminophosphorylamine |
https://en.wikipedia.org/wiki/Phosphoramide
No comments:
Post a Comment