SR-142948 is a drug used in scientific research which is a non-peptide antagonist selective for the neurotensin receptors, although not selective between subtypes.[1]

https://en.wikipedia.org/wiki/SR-142948
GRL-0617 is a drug which is one of the first compounds discovered that acts as a selective small-molecule inhibitor of the protease enzyme papain-like protease(PLpro) found in some pathogenic viruses, including the coronavirus SARS-CoV-2. It has been shown to inhibit viral replication in vitro.[1][2][3][4]
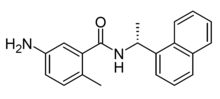
https://en.wikipedia.org/wiki/GRL-0617
GRN-529 is a drug that was developed by Wyeth as a negative allosteric modulatorof the metabotropic glutamate receptor 5 (mGluR5).[1]
A study conducted by Pfizer found that GRN-529 reduced repetitive behaviors without sedation and partially increased sociability in mouse models of autism. [2]
Another study conducted by Pfizer found a therapeutically relevant effect in animal models of depression. It is theorized to work by reducing glutamate receptor hyperactivity.[3]

https://en.wikipedia.org/wiki/GRN-529
TORT/TRAFF DRUG
Hippuric acid (Gr. hippos, horse, ouron, urine) is a carboxylic acid and organic compound. It is found in urine and is formed from the combination of benzoic acidand glycine. Levels of hippuric acid rise with the consumption of phenolic compounds(such as fruit juice, tea and wine). The phenols are first converted to benzoic acid, and then to hippuric acid and excreted in urine.[1]
Hippuric acid crystallizes in rhombic prisms which are readily soluble in hot water, melt at 187 °C, and decompose at about 240 °C. High concentrations of hippuric acid may also indicate a toluene intoxication; however, scientists have called this correlation into question because there are other variables that affect levels of hippuric acid.[2] When many aromatic compounds such as benzoic acid and tolueneare taken internally, they are converted to hippuric acid by reaction with the amino acid, glycine.

https://en.wikipedia.org/wiki/Hippuric_acid
BRANCH DRUG AT BENZOIC ACID AND GLYCINE AND CARBOXYLIC ACID
Fallypride is a high affinity dopamine D2/D3 receptor antagonist used in medical research,[1] usually in the form of fallypride (18F) as a positron emission tomography(PET) radiotracer in human studies.[2][3]
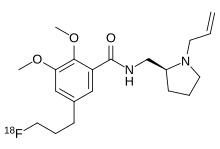
https://en.wikipedia.org/wiki/Fallypride
TORT/TRAFF DRUG
Fluopicolide is a fungicide used in agriculture to control diseases caused by oomycetes such as late blight of potato. It is classed as an acylpicolide and its chemical name is 2,6-dichloro-N-{[3-chloro-5-(trifluoromethyl)pyridin-2-yl]methyl}benzamide.[1] The precise mode of action is not known, but it is thought to act by affecting spectrin-like proteins in the cytoskeleton of oomycetes.[1][2] This mode of action differs from other available fungicides used to control oomycetes and it can inhibit the growth of strains that are resistant to phenylamides, strobilurin, dimethomorph and iprovalicarb.[1] It has some systemic activity as it moves through the xylem towards the tips of stems, but does not get transported to the roots.[2] It affects the motility of zoospores, the germination of cysts, the growth of the myceliumand sporulation.[3] Bayer CropScience developed the compound and it was first released as a commercial product in 2006.[4]

https://en.wikipedia.org/wiki/Fluopicolide
Fluopyram is a fungicide and nematicide used in agriculture.[2][3] It is used to control fungal diseases such as gray mold (Botrytis), powdery mildew, apple scab, Alternaria, Sclerotinia, and Monilinia. It is an inhibitor of succinate dehydrogenase.[4]
In 2012, it was approved by the U.S. Environmental Protection Agency[4] and in 2013 it was approved in the EU for use as an active ingredient in pesticides.[5]

https://en.wikipedia.org/wiki/Fluopyram
Etamivan (INN, or ethamivan (USAN); trade names Analepticon, Emivan, and Vandid) is a respiratory stimulant drug[1] related to nikethamide. It was mainly used in the treatment of barbiturate overdose[2] and chronic obstructive pulmonary disease,[3] but has now largely fallen into disuse.
Adverse effects which are common to the respiratory stimulant class include sneezing, coughing, and laryngospasm when infused too rapidly. More serious adverse events include muscle twitching, tremors, and convulsions. The dose to treat barbiturate intoxication or carbon dioxide narcosis in adults ranges from 0.5 mg/kg to 5.0 mg/kg, infused intravenously over several minutes. Epilepsy and the use of monoamine oxidase inhibitors or other adrenergic drugs are contraindications.[4]

https://en.wikipedia.org/wiki/Etamivan
Entinostat, also known as SNDX-275 and MS-275, is a benzamide histone deacetylase inhibitor undergoing clinical trials for treatment of various cancers.[1]
Entinostat inhibits class I HDAC1 and HDAC3 with IC50 of 0.51 μM and 1.7 μM, respectively.[2]
Syndax pharmaceuticals currently holds the rights to Entinostat and recently received $26.6 million in funds to advance treatments of resistant cancers using epigenetic tools.[3]
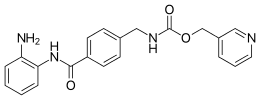
https://en.wikipedia.org/wiki/Entinostat
RWJ-394674 is a drug that is used in scientific research. It is a potent, orally active analgesic drug that produces little respiratory depression. RWJ-394674 itself is a potent and selective agonist for δ-opioid receptors, with a Ki of 0.24 nM at δ and 72 nM at μ. However once inside the body, RWJ-394674 is dealkylated to its monodesethyl metabolite RWJ-413216, which is a potent agonist at the μ-opioid receptor and has less affinity for δ (Ki 0.26 nM at μ, 46.7 nM at δ). The effect of RWJ-394674 when administered in vivo thus produces potent agonist effects at both μ and δ receptors through the combined actions of the parent drug and its active metabolite, with the δ-agonist effects counteracting the respiratory depression from the μ-opioid effects, and the only prominent side-effect being sedation.[1]
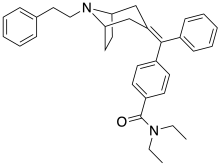
https://en.wikipedia.org/wiki/RWJ-394674
TORT/TRAFF DRUG ANEST
Roflumilast, sold under the trade name Daxas among others, is a drug that acts as a selective, long-acting inhibitor of the enzyme phosphodiesterase-4 (PDE-4). It has anti-inflammatory effects and is used as an orally administered drug for the treatment of inflammatory conditions of the lungs such as chronic obstructive pulmonary disease (COPD).[6][7][8][9]
In June 2010, it was approved in the European Union for severe COPD associated with chronic bronchitis.[3][10] In February 2011, it gained FDA approval in the United States for reducing COPD exacerbations.[11][12]
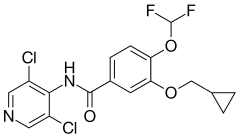
https://en.wikipedia.org/wiki/Roflumilast
Ro 44-3888 is a non-peptidic, selective and reversible glycoprotein IIb/IIIa inhibitor. It is the active metabolite of sibrafiban. It was being investigated as a treatment for heart conditions, including myocardial infarctions. The development of Ro 44-3888 and sibrafiban was discontinued in 1999 following unfavorable Phase III efficacy data.
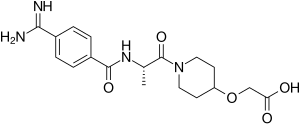
https://en.wikipedia.org/wiki/Ro-44-3888
Rebamipide, an amino acid derivative of 2-(1H)-quinolinone, is used for mucosalprotection,[1] healing of gastroduodenal ulcers, and treatment of gastritis.[2] It works by enhancing mucosal defense, scavenging free radicals,[3] and temporarily activating genes encoding cyclooxygenase-2.[4]
Studies have shown that rebamipide can fight the damaging effects of NSAIDs on the GIT mucosa,[5] and more recently, the small intestine, but not for naproxen-induced gastric damage.[6]

https://en.wikipedia.org/wiki/Rebamipide
Rafoxanide is a salicylanilide used as an anthelmintic.[1] It is most commonly used in ruminant animals to treat adult liver flukes of the species Fasciola hepatica and Fasciola gigantica.[1]

https://en.wikipedia.org/wiki/Rafoxanide
Radotinib (INN; trade name Supect), and sometimes referred to by its investigational name IY5511, is a drug for the treatment of different types of cancer, most notably Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia(CML)[1] with resistance or intolerance of other Bcr-Abl tyrosine-kinase inhibitors, such as patients resistant or intolerant to imatinib.
Radotinib is being developed by Ilyang Pharmaceutical Co., Ltd of South Korea[2]and is co-marketed by Daewoong Pharmaceutical Co. Ltd, in South Korea.[3]Radotinib completed a multi-national Phase II clinical trial study in 2012[4] and in August 2011, Ilyang initiated a Phase III, multinational, multi-center, open-label, randomized study for first-line indication.[5] Its mechanism of action involves inhibition of the Bcr-Abl tyrosine kinase and of platelet-derived growth factor receptor(PDGFR).[6]

https://en.wikipedia.org/wiki/Radotinib
Dirlotapide is a drug used to treat obesity in dogs.[1] It is manufactured by Pfizer and Zoetis and marketed under the brand name Slentrol.
It works as a gut-selective microsomal triglyceride transfer protein (MTTP or MTP) inhibitor.[2] This blocks the assembly and release of lipoproteins into the bloodstream, thereby reducing fat absorption. It also elicits a satiety signal from lipid-filled cells lining the intestine. (obesogenic)

https://en.wikipedia.org/wiki/Dirlotapide
Dinitolmide (or zoalene) is a fodder additive for poultry, used to prevent coccidiosisinfections.[2] It is sold under trade names such as Coccidine A, Coccidot, and Zoamix.
Dinitolmide is usually added to feed in doses of 125 ppm (preventive) or 250 ppm (curative). It is a broad-spectrum anticoccidial drug,[2] preventing seven main strains of Eimeria coccidium. It leaves no residues in tissues.[citation needed] It can be also used to prevent coccidiosis of domestic rabbits.
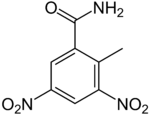
https://en.wikipedia.org/wiki/Dinitolmide
Diflubenzuron is an insecticide of the benzoylurea class.[2] It is used in forest management and on field crops[3] to selectively control insect pests, particularly forest tent caterpillar moths, boll weevils, gypsy moths, and other types of moths.[1] It is a widely used larvicide in India for control of mosquito larvae by public health authorities. Diflubenzuron is approved by the WHO Pesticide Evaluation Scheme.[1]
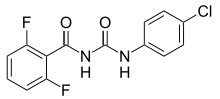
https://en.wikipedia.org/wiki/Diflubenzuron
Dexloxiglumide is a drug which acts as a cholecystokinin antagonist, selective for the CCKA subtype. It inhibits gastrointestinal motility and reduces gastric secretions, and despite older selective CCKA antagonists such as lorglumide and devazepidehaving had only limited success in trials and ultimately never making it into clinical use, dexloxiglumide is being investigated as a potential treatment for a variety of gastrointestinal problems including irritable bowel syndrome,[1] dyspepsia,[2]constipation[3] and pancreatitis,[4][5] and has had moderate success so far although trials are still ongoing.

https://en.wikipedia.org/wiki/Dexloxiglumide
PNU-282,987 is a drug that acts as a potent and selective agonist for the α7 subtype of neural nicotinic acetylcholine receptors.[1][2] In animal studies, it shows nootropiceffects, and derivatives may be useful in the treatment of schizophrenia,[3][4] although PNU-282,987 is not suitable for use in humans because of excessive inhibition of the hERG antitarget.[5] PNU-282987 has been shown to initiate signaling that leads to adult neurogeneis in mammals.[6]

https://en.wikipedia.org/wiki/PNU-282,987
Pranlukast (brand name Onon, オノン) is a cysteinyl leukotriene receptor-1 antagonist. This drug works similarly to Merck & Co.'s montelukast (Singulair). It is widely used in Japan.
Medications of this class, which go under a variety of names according to whether one looks at the American, British or European system of nomenclature, have as their primary function the antagonism of bronchospasm caused, principally in asthmatics, by an allergic reaction to accidentally or inadvertently encountered allergens.[2]
Medications of this group are normally used as an adjunct to the standard therapy of inhaled steroids with inhaled long- and/or short-acting beta-agonists. There are several similar medications in the group; all appear to be equally effective. Pranlukast is also reported as potential inhibitor of Mycobacterium tuberculosis infection in experimental models.[3]

https://en.wikipedia.org/wiki/Pranlukast
Ochratoxin A—a toxin produced by different Aspergillus and Penicillium species — is one of the most-abundant food-contaminating mycotoxins.[1] It is also a frequent contaminant of water-damaged houses and of heating ducts.[2][3] Human exposure can occur through consumption of contaminated food products, particularly contaminated grain and pork products, as well as coffee, wine grapes, and dried grapes.[4][5][6] The toxin has been found in the tissues and organs of animals, including human blood and breast milk.[7] Ochratoxin A, like most toxic substances, has large species- and sex-specific toxicological differences.[5]

https://en.wikipedia.org/wiki/Ochratoxin_A
Cyantraniliprole is an insecticide of the ryanoid class, specifically a diamide insecticide (IRAC MoA group 28).[1] It is approved for use in the United States, Canada, China, and India.[2] Because of its uncommon mechanism of action as a ryanoid, it has activity against pests such as Diaphorina citri that have developed resistance to other classes of insecticides.[3] Cyantraniliprole is highly toxic to bees, which resulted in registration of its use as a pesticide being delayed in the USA.[4]
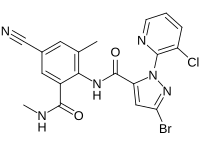
https://en.wikipedia.org/wiki/Cyantraniliprole
CDPPB is a drug used in scientific research which acts as a positive allosteric modulator selective for the metabotropic glutamate receptor subtype mGluR5.[1][2][3] It has antipsychotic effects in animal models,[4] and mGluR5 modulators are under investigation as potential drugs for the treatment of schizophrenia,[5] as well as other applications.[6][7]

https://en.wikipedia.org/wiki/CDPPB
TORT/TRAFF DRUG
Cariporide is a selective Na+/H+ exchange inhibitor. Cariporide has been shown to actively suppress the cell death caused by oxidative stress.[1]
Cariporide is a potent NHE1 inhibitor is may also be useful for treatment of cancer.[2]

https://en.wikipedia.org/wiki/Cariporide
TRAFF/TORT DRUG
Binimetinib, also known as Mektovi and ARRY-162, is an anti-cancer small molecule that was developed by Array Biopharma to treat various cancers.[1]Binimetinib is a selective inhibitor of MEK, a central kinase in the tumor-promoting MAPK pathway.[2] Inappropriate activation of the pathway has been shown to occur in many cancers.[2] In June 2018 it was approved by the FDA in combination with encorafenib for the treatment of patients with unresectable or metastatic BRAF V600E or V600K mutation-positive melanoma.[3][4]
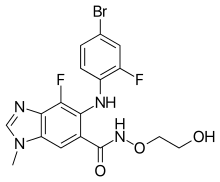
https://en.wikipedia.org/wiki/Binimetinib
Bezafibrate (marketed as Bezalip and various other brand names) is a fibrate drugused as a lipid-lowering agent to treat hyperlipidaemia. It helps to lower LDL cholesterol and triglyceride in the blood, and increase HDL.
It was patented in 1971 and approved for medical use in 1978.[1]

https://en.wikipedia.org/wiki/Bezafibrate
Betamipron (INN) or N-benzoyl-β-alanine is a chemical compound which is used together with panipenem[1] to inhibit panipenem uptake into the renal tubule and prevent nephrotoxicity.[2]

https://en.wikipedia.org/wiki/Betamipron
Benmoxin (trade names Neuralex, Nerusil), also known as mebamoxine, is an irreversible and nonselective monoamine oxidase inhibitor (MAOI) of the hydrazineclass.[1][2] It was synthesized in 1967 and was subsequently used as an antidepressant in Europe, but is now no longer marketed.[1][2]

https://en.wikipedia.org/wiki/Benmoxin
BDTH2 (also called BDET and BDETH2; trade names B9, MetX and OSR#1) is an organosulfur compound that is used as a chelation agent.[2] It is a colourless solid. The molecule consists of two thiol groups and linked via a pair of amide groups.[3

https://en.wikipedia.org/wiki/BDTH2
Balanol is a fungal metabolite produced by the fungus Verticillium balanoides.[1] It is a potent inhibitor of the serine/threonine kinases protein kinase A (PKA) and protein kinase C (PKC), binding in a similar manner with that of ATP.[2] Balanol was discovered in 1993 in the search for novel inhibitors of PKC, a member of a family of serine/threonine kinases whose overactivation is associated with numerous human diseases of signal transduction including cancer. However, much of the research on balanol focuses on how chemical modifications of the molecular structure affect binding to PKA.[3] Indeed, balanol, its chemically altered analogs, and their interactions with PKA in particular are used to illuminate the roles of selectivity and protein flexibility in the inhibition of kinases. For instance, the X-ray crystal structure of balanol in complex with PKA was used in order to confer selectivity and to improve pharmacological efficacy of inhibitors of the H. sapiens Akt (PKB), another serine/threonine protein kinase implicated in the proper functioning of many cellular processes.[4]
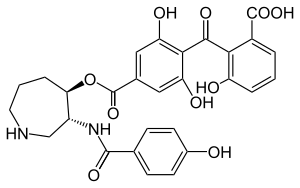
https://en.wikipedia.org/wiki/Balanol
Methylhippuric acid is a carboxylic acid and organic compound. Methylhippuric acid has three isomers. The isomers include 2-, 3-, and 4-methylhippuric acid.[1]
Methylhippuric acids are metabolites of the isomers of xylene.[1][2] The presence of methylhippuric acid can be used as a biomarker to determine exposure to xylene.[2][3]

https://en.wikipedia.org/wiki/Methylhippuric_acid
Mersalyl (Mersal) is an organomercury compound[1] and mercurial diuretic. It is only rarely used as a drug, having been superseded by diuretic medications that do not contain mercury and are therefore less toxic. It features a Hg(II) centre. Mersalyl was originally adapted from calomel (Hg2Cl2), a diuretic discovered by Paracelsus.
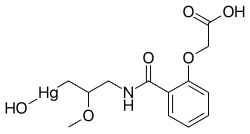
https://en.wikipedia.org/wiki/Mersalyl
Mosapride is a gastroprokinetic agent that acts as a selective 5HT4 agonist. The major active metabolite of mosapride, known as M1, additionally acts as a 5HT3antagonist,[1] which accelerates gastric emptying throughout the whole of the gastrointestinal tract in humans,[2] and is used for the treatment of gastritis, gastroesophageal reflux disease, functional dyspepsia[3] and irritable bowel syndrome.[4] It is recommended to be taken on an empty stomach (i.e. at least one hour before food or two hours after food).[5]
In addition to its prokinetic properties, mosapride also exerts anti-inflammatory effects on the gastrointestinal tract which may contribute to some of its therapeutic effects.[6] Mosapride also promotes neurogenesis in the gastrointestinal tract which may prove useful in certain bowel disorders.[7][8] The neurogenesis is due to mosapride's effect on the 5-HT4 receptor where it acts as an agonist.[9]
Its common side effects include dry mouth, abdominal pain, dizziness, headache, insomnia, malaise, nausea, diarrhea and sometimes constipation.[3][10] Unlike some other prokinetic agents, mosapride has little effect on potassium channels, no effect on hERG transfected cells, and no effect on cardiovascular function that could be detected in tests on humans.[1][11] Due to the pharmacokinetics of mosapride, it would take 1,000–3,000 times the therapeutic dose to elicit cardiovascular effects.[12]

https://en.wikipedia.org/wiki/Mosapride
MPPF, with the full name 2'-methoxyphenyl-(N-2'-pyridinyl)-p-fluoro-benzamidoethyipiperazine, is a compound that binds to the serotonin-1A receptor. Labeled with fluorine-18 it has been used as a radioligand with positron emission tomography.[1] It has, e.g., been used to examine the difference in neuroreceptor binding in the human brain across sex and age.[2]

https://en.wikipedia.org/wiki/MPPF
Nelfinavir (NFV; brand name Viracept) is an antiretroviral drug used in the treatment of the human immunodeficiency virus (HIV). Nelfinavir belongs to the class of drugs known as protease inhibitors (PIs) and like other PIs is almost always used in combination with other antiretroviral drugs. Nelfinavir has been shown to treat SARS-coronavirus, and is being tested to treat COVID-19.
Nelfinavir is an orally bioavailable human immunodeficiency virus HIV-1 proteaseinhibitor (Ki=2nM) and is widely prescribed in combination with HIV reverse transcriptase inhibitors for the treatment of HIV infection.[2]
It was patented in 1992 and approved for medical use in 1997.[3]
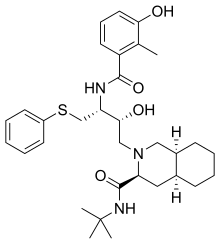
https://en.wikipedia.org/wiki/Nelfinavir
Lufenuron is the active ingredient in the veterinary flea control medication program, and one of the two active ingredients in the flea, heartworm, ringworm and anthelmintic medicine milbemycin oxime/lufenuron (Sentinel).
Lufenuron is stored in the animal's body fat and transferred to adult fleas through the host's blood when they feed. Adult fleas transfer it to their growing eggs through their blood, and to hatched larvae feeding on their excrement. It does not kill adult fleas.[citation needed]
Lufenuron, a benzoylurea pesticide, inhibits the production of chitin in insects. Without chitin, a larval flea will never develop a hard outer shell (exoskeleton). With its inner organs exposed to air, the insect dies from dehydration soon after hatching or molting (shedding its old, smaller shell).[citation needed]
Lufenuron is also used to fight fungal infections, since fungus cell walls are about one third chitin.[1]
Lufenuron is also sold as an agricultural pesticide for use against lepidopterans, eriophyid mites, and western flower thrips. It is an effective antifungal in plants.[2]
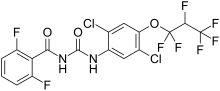
https://en.wikipedia.org/wiki/Lufenuron
Lorglumide (CR-1409) is a drug which inhibits gastrointestinal motility and reduces gastric secretions, acting as a cholecystokinin antagonist,[1] with fairly high selectivity for the CCKA subtype.[2] It has been suggested as a potential treatment for a variety of gastrointestinal problems including stomach ulcers, irritable bowel syndrome, dyspepsia, constipation and pancreatitis, as well as some forms of cancer, but animal and human testing has produced inconsistent results and no clear therapeutic role has been established, although it is widely used in scientific research.[3][4][5][6]

https://en.wikipedia.org/wiki/Lorglumide
Kaitocephalin is a non-selective ionotropic glutamate receptor antagonist, meaning it blocks the action of the neurotransmitter glutamate. It is produced by the fungusEupenicillium shearii. Although similar molecules have been produced synthetically, kaitocephalin is the only known naturally occurring glutamate receptor antagonist.[1][2] There is some evidence that kaitocephalin can protect the brain and central nervous system, so it is said to have neuroprotective properties. Kaitocephalin protects neurons by inhibiting excitotoxicity, a mechanism which causes cell death by overloading neurons with glutamate.[3] Because of this, it is of interest as a potential scaffold for drug development. Drugs based on kaitocephalin may be useful in treating neurological conditions, including Alzheimer's, amyotrophic lateral sclerosis (ALS), and stroke.[4]

https://en.wikipedia.org/wiki/Kaitocephalin
Itopride (INN) (brand name Ganaton) is a prokinetic benzamide derivative. These drugs inhibit dopamine and acetylcholine esterase enzyme and have a gastrokineticeffect.[3] Itopride is indicated for the treatment of functional dyspepsia and other gastrointestinal conditions.[4] It is a combined D2 receptor antagonist and acetylcholinesterase inhibitor.[5][6]
Itopride is not currently approved by the U.S. Food and Drug Administration (FDA) for use in the United States, nor is it yet approved in the United Kingdom. This may explain the apparent lack of patient information available in English compared to other similar classes of medication.
Itopride has no effect on potassium channels.[16]
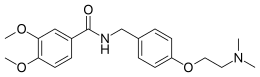
https://en.wikipedia.org/wiki/Itopride
INTERROGATION DRUG.
[PRUSS SSF bf. 1900s ~ DRYDEHY ANOXIA-ChPh Depleted Dop Sig KO D-NIC-P-A.T-Gene, Dip Phos, Alkalize]
(USA D2 ATP/MC/ER/P/etc. @ ACh/ache.r/achei/etc. BLC Rev Irrev noncomp antagon) enzy rec subst gene prod diffu compo fl-fo ion time sites partial-bind trigger (gene or substrate or site bind) rev irrev noncomp ant
(USA DS NR ANT GKO SLUDGE WT ACh) AChE Inhi, analog to fix site ACh R, bind. Gene Upreg AChE, AChR and ACh. Knock Enzyme. Gen Mod ACh analog pathway trigger receptor with substrate, SLUDGE DEMENT.
Acetylcholine (ACh) is an organic chemical that functions in the brain and body of many types of animals (including humans) as a neurotransmitter—a chemical message released by nerve cells to send signals to other cells, such as neurons, muscle cells and gland cells.[1] Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Parts in the body that use or are affected by acetylcholine are referred to as cholinergic. Substances that increase or decrease the overall activity of the cholinergic system are called cholinergics and anticholinergics, respectively.
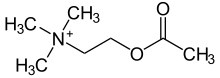
| Clinical data | |
|---|---|
| Other names | ACh |
| Physiological data | |
| Source tissues | motor neurons, parasympathetic nervous system, brain |
| Target tissues | skeletal muscles, brain, many other organs |
| Receptors | nicotinic, muscarinic |
| Agonists | nicotine, muscarine, cholinesterase inhibitors |
| Antagonists | tubocurarine, atropine |
| Precursor | choline, acetyl-CoA |
| Biosynthesis | choline acetyltransferase |
| Metabolism | acetylcholinesterase |
https://en.wikipedia.org/wiki/Acetylcholine
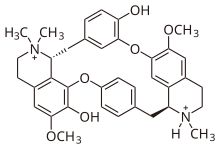
Tubocurarine (also known as d-tubocurarine or DTC) is a toxic alkaloid historically known for its use as an arrow poison. In the mid-1900s, it was used in conjunction with an anesthetic to provide skeletal muscle relaxation during surgery or mechanical ventilation. It is now rarely used as an adjunct for clinical anesthesia because safer alternatives, such as cisatracurium and rocuronium, are available.
https://en.wikipedia.org/wiki/Tubocurarine_chloride
3CLpro-1 is an antiviral drug related to rupintrivir which acts as a 3CL protease inhibitor and was originally developed for the treatment of human enterovirus 71. It is one of the most potent of a large series of compounds developed as inhibitors of the viral enzyme 3CL protease, with an in vitro IC50 of 200 nM. It also shows activity against coronavirus diseases such as SARS and MERS, and is under investigation as a potential treatment agent for the viral disease COVID-19.[1][2][3][4][5][6][7]

https://en.wikipedia.org/wiki/3CLpro-1
Theaflavin digallate (TFDG) is an antioxidant natural phenol found in black tea, and a theaflavin derivative.

https://en.wikipedia.org/wiki/Theaflavin_digallate
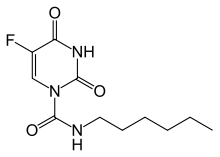
Carmofur (INN) or HCFU (1-hexylcarbamoyl-5-fluorouracil) is a pyrimidine analogueused as an antineoplastic agent. It is a derivative of fluorouracil, being a lypophilic-masked analog of 5-FU that can be administered orally.[1]
https://en.wikipedia.org/wiki/Carmofur
Sivelestat (INN, research name ONO 5046, marketed as Elaspol) is an inhibitor of human neutrophil elastase.[1]
It is used in the treatment of acute respiratory failure[2] and preliminary studies show it may also improve neuropathic pain.[3]

https://en.wikipedia.org/wiki/Sivelestat
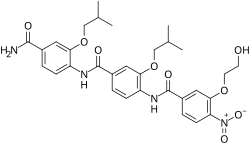
ERX-11, also known as ERα coregulator-binding modulator-11, is a novel antiestrogen and experimental hormonal antineoplastic agent which is being researched for the potential treatment of estrogen receptor-positive breast cancer.[1][2] It is not a competitive antagonist of the estrogen receptor (ER) like conventional antiestrogens such as tamoxifen or fulvestrant; instead of binding to the ligand-binding site of the ER, ERX-11 interacts with a different part of the ERα and blocks protein–protein interactions of the ERα with coregulators that are necessary for the receptor to act and regulate gene expression.[1][2] It was designed to bind to the coregulator binding region of the ERα and inhibit the ERα/coactivator interaction, although its precise binding site and mode of action have yet to be fully elucidated and understood.[1][2][3][4] Nonetheless, it is clear that ERX-11 binds within the AF-2 domain of the ERα.[1]
https://en.wikipedia.org/wiki/ERX-11
Proglumide (Milid) is a drug that inhibits gastrointestinal motility and reduces gastric secretions. It acts as a cholecystokinin antagonist,[1] which blocks both the CCKA and CCKB subtypes.[2] It was used mainly in the treatment of stomach ulcers,[3][4]although it has now been largely replaced by newer drugs for this application.
An interesting side effect of proglumide is that it enhances the analgesia produced by opioid drugs,[5] and can prevent or even reverse the development of tolerance to opioid drugs.[6][7] This can make it a useful adjuvant treatment to use alongside opioid drugs in the treatment of chronic pain conditions such as cancer, where opioid analgesics may be required for long periods and development of tolerance reduces clinical efficacy of these drugs.[8][9]

https://en.wikipedia.org/wiki/Proglumide
Procarbazine is a chemotherapy medication used for the treatment of Hodgkin's lymphoma and brain cancers.[1] For Hodgkin's it is often used together with chlormethine, vincristine, and prednisone while for brain cancers such as glioblastoma multiforme it is used with lomustine and vincristine.[1] It is typically taken by mouth.[1]
Common side effect include low blood cell counts and vomiting.[1] Other side effects include tiredness and depression.[2][3] It is not recommended in people with severe liver or kidney problems.[4] Use in pregnancy is known to harm the baby.[1]Procarbazine is in the alkylating agents family of medication.[1] How it works is not clearly known.[1]
Procarbazine was approved for medical use in the United States in 1969.[1] It is on the World Health Organization's List of Essential Medicines, the safest and most effective medicines needed in a health system.[5] In the United Kingdom a month of treatment cost the NHS 450 to 750 pounds.[4]

https://en.wikipedia.org/wiki/Procarbazine
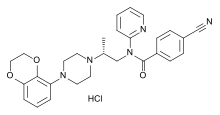
Lecozotan is an investigational drug by Wyeth tested for improvement of cognitive functions of Alzheimer's disease patients.[1] As of June 2008, the first Phase III clinical trial has been completed.[2]
Method of action[edit]
Lecozotan is a competitive, selective 5-HT1A receptor antagonist[3] which enhances the potassium-stimulated release of acetylcholine and glutamate.[4]
https://en.wikipedia.org/wiki/Lecozotan
Pages in category "Catechol ethers"
The following 68 pages are in this category, out of 68 total. This list may not reflect recent changes (learn more).
0–9
2C-O-4
A
Acetyldihydrocodeine
Acotiamide
Aliskiren
Amosulalol
Antrocamphin B
Apremilast
Aspidophytine
Asterric acid
B
Befunolol
Benzamidenafil
Benzhydrocodone
Benzquinamide
Bevantolol
Bosentan
Bosutinib
Brucine
Bufetolol
Bunazosin
Buprenorphine-3-glucuronide
C
Carbofuran
Carbosulfan
Carvedilol
Cediranib
Ciladopa
Cilomilast
Cinepazet
Codeine
Codeine methylbromide
Codeinone
Codoxime
Conorfone
Cryogenine
Cutamesine
Cyclotriveratrylene
D
Decoquinate
Denopamine
Devapamil
Dibutyrylmorphine
Dihydrocodeine
Dihydrotetrabenazine
Dimeditiapramine
3,4-Dimethoxyphenethylamine
3,4-Dimethoxystyrene
Dipivefrine
Donepezil
E
Ensaculin
G
Guacetisal
Guaifenesin
I
Itopride
L
Levomoprolol
M
Mebeverine
Methocarbamol
Methoxsalen
Moguisteine
Moprolol
N
Nisoxetine
O
Oxprenolol
Oxycodone
Oxypertine
P
Pacrinolol
Papaverine
Piperolactam A
Prazosin
Propoxur
S
Scoulerine
V
Verapamil
Vesnarinone
https://en.wikipedia.org/wiki/Category:Catechol_ethers
Subcategories
This category has the following 26 subcategories, out of 26 total.
0–9
2-phenoxyethanamines (10 P)
2-Quinolone ethers at the benzene ring (1 C, 4 P)
3-(4-methoxyphenyl)-2-oxazolidinones (5 P)
4-Hydroxybiphenyl ethers (5 P)
B
Benzofuran ethers at the benzene ring (15 P)
Benzofurans (4 C, 26 P)
C
Catechol ethers (5 C, 68 P)
Chromenes (6 C, 25 P)
D
Diphenyl ethers (4 C, 29 P)
H
Hydroquinone ethers (2 C, 13 P)
I
Indole ethers at the benzene ring (1 C, 24 P)
M
Melatonin (1 C, 5 P)
N
N-(2-methoxyphenyl)piperazines (13 P)
Naphthol ethers (18 P)
O
O-Methylated isoflavones (16 P)
O-Methylated phenols (4 C, 30 P)
P
Para-Methoxyphenylpiperazines (9 P)
Phenol esters (2 C, 64 P)
Phenoxyacetic acids (1 C, 6 P)
Phenoxypropanolamines (1 C, 8 P)
Phenyl sulfonate ethers (3 P)
Piceol ethers (15 P)
R
Resorcinol ethers (2 C, 25 P)
S
Salicylamide ethers (1 C, 14 P)
Salicylyl ethers (8 P)
V
Vanilloids (1 C, 13 P)
Pages in category "Phenol ethers"
The following 200 pages are in this category, out of approximately 683 total. This list may not reflect recent changes (learn more).
(previous page) (next page)
*
Phenol ether
0–9
4-MeO-PCP
7-PET
A
A-971432
Acitretin
Aclidinium bromide
Advantame
Afatinib
Alclofenac
Aleglitazar
Allyl phenyl ether
Alprenoxime
Ambucetamide
1-Aminomethyl-5-methoxyindane
Andarine
Anethole trithione
Anisic acid
Anisole
Anisomycin
Anisoyl chloride
Anisyl acetate
Anisyl alcohol
Anitrazafen
Apixaban
Arformoterol
Aristolochic acid
Astemizole
Atomoxetine
Avanafil
Azadipyrromethene
Para-Azoxyanisole
Azoxystrobin
B
Balofloxacin
BAPTA
Bazedoxifene
Bemotrizinol
Benzethonium chloride
Benzylmorphine
Bifemelane
Bis-HPPP
Bisphenol A diglycidyl ether
BMY-7378
Boldine
Bolinaquinone
BP-897
2-Bromoanisole
4-Bromoanisole
Bromoketamine
Bufexamac
C
Carbestrol
Carmoterol
Caroverine
Ceritinib
Chlorophetanol
Chlorotrianisene
Chlorphenesin carbamate
Choline m-bromophenyl ether
Ciglitazone
Cimoxatone
Cinaciguat
Cinchocaine
Cirazoline
Climbazole
Clorgiline
Coenzyme Q10
Coomassie Brilliant Blue
CP-94253
Creosol
Para-Cresidine
Cresidinesulfonic acid
Croconazole
Curculigoside
Curculigoside A
Cyprodime
D
Damascenine
Damnacanthal
Dapagliflozin
Dapagliflozin/metformin
Dapagliflozin/saxagliptin/metformin
Dapagliflozin/saxagliptin
Desmethylchlorotrianisene
Dextromethorphan
Dibrompropamidine
2,4-DB
Dichlorprop
Diclofensine
Dihydroquinidine
Dihydroquinine
Diltiazem
Dimethoxyamphetamine
2,6-Dimethoxybenzoquinone
2,4-Dinitroanisole
2,4-Dinitrophenylmorphine
Diosmetinidin
Dioxybenzone
Diproteverine
Direct Blue 1
Disperse Red 11
DMCM
Dofetilide
Domiphen bromide
Doravirine
Doxazosin
Dronedarone
Drotebanol
E
EA-2012
EA-2054
EA-2098
EA-2613
Ecastolol
EEE (psychedelic)
EEM (psychedelic)
Efaproxiral
Efloxate
Elemicin
Eletefine
Embutramide
EME (psychedelic)
Emicerfont
Emixustat
EMM (psychedelic)
Encainide
Enclomifene
Enilospirone
Enobosarm
Enprostil
Erastin
Erlotinib
Ertugliflozin
Escaline
Esomeprazole
Esperamicin
Esreboxetine
Estragole
Etafenone
Etalocib
Etamivan
Ethacridine lactate
Ethamoxytriphetol
Ethenzamide
Ethoxyquin
Ethoxzolamide
Ethyl phenyl ether
EMDT
4-Ethylguaiacol
Ethylmorphine
Ethylvanillin
Eticlopride
Etofamide
Etofibrate
Etomoxir
Etonitazene
Etoposide
Etretinate
Eugenin
Eugenitin
Evoxine
Ezlopitant
F
Fabomotizole
Falipamil
Fallypride
Famoxadone
Fantofarone
Febuxostat
Femoxetine
Fenoprop
Fenoxazoline
Fenoxycarb
Fenvalerate
Ferujol
Fexinidazole
FGI-104
Filaminast
Finerenone
Fipexide
Flavokavain A
Flavokavain B
Flavokavain C
Flecainide
Flocoumafen
Fluanisone
Flumizole
Fluo-3
Fluo-4
4'-Fluorocannabidiol
Fluoroethyl-L-tyrosine (18F)
Fluoxetine
Fluvalinate
Formoterol
Fospropofol
G
Gabazine
Gadoxetic acid
Galantamine
Gallamine triethiodide
Ganesha (psychedelic)
Garenoxacin
Gatifloxacin
GBLD-345
Gedocarnil
Gefitinib
https://en.wikipedia.org/wiki/Category:Phenol_ethers
https://en.wikipedia.org/wiki/Category:Benzamides
No comments:
Post a Comment