β-Propiolactone is an organic compound of the lactone family, with a four-membered ring. It is a colorless liquid with a slightly sweet odor, highly soluble in water and miscible with ethanol, acetone, diethyl ether and chloroform.[2][3] The word propiolactone usually refers to this compound, although it may also refer to α-propiolactone.

| Names | |
|---|---|
| Preferred IUPAC name Oxetan-2-one | |
| Other names Propiolactone β-Propiolactone 2-Oxetanone 3-Hydroxypropanoic acid lactone |
β-Propiolactone is prepared industrially by the reaction of formaldehyde and ketenein the presence of aluminium- or zinc chloride as catalyst:[4]
In the research laboratory, propiolactones have been produced by the carbonylationof epoxides.[5]
β-Propiolactone readily polymerizes even at room temperature.[citation needed]
It reacts with many nucleophiles in a ring-opening reactions. With water hydrolysisoccurs to produce 3-hydroxypropionic acid (hydracryclic acid). Ammonia gives the β-alanine, which is a commercial process.[4]
Propiolactone was once widely produced as an intermediate in the production of acrylic acid and its esters. That application has been largely displaced in favor of safer and less expensive alternatives. β-Propiolactone is an excellent sterilizing and sporicidal agent, but its carcinogenicity precludes that use.[2] It is used to inactivate a wide variety of viruses,[6] for example as a step in vaccine production.[7] The principal use of propiolactone is an intermediate in the synthesis of other chemical compounds.[4]
Acidovorax sp., Variovorax paradoxus, Sphingomonas paucimobilis, Rhizopus delemar and thermophilic Streptomyces sp. can degrade β-propiolactone.[citation needed]
https://en.wikipedia.org/wiki/Beta-Propiolactone
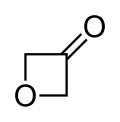
3-Oxetanone, also called oxetan-3-one or 1,3-epoxy-2-propanone, is a chemical compound with formula C3H4O2. It is the ketone of oxetane, and an isomer of β-propiolactone.
3-Oxetanone is a liquid at room temperature, that boils at 140 °C. It is a specialty chemical,[1][2] used for research in the synthesis of other oxetanes of pharmacological interest.[3][4] Oxetan-3-one also has been the object of theoretical studies.[5][6]
| Names | |
|---|---|
| Preferred IUPAC name Oxetan-3-one | |
| Other names 1,3-Epoxy-2-propanone 1,3-Epoxypropanone 1,3-Epoxy-2-propan-2-one |
https://en.wikipedia.org/wiki/3-Oxetanone
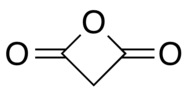
Malonic anhydride or oxetane-2,4-dione is an organic compound with chemical formula C3H2O3 or CH2(CO)2O. It can be viewed as the anhydride of malonic acid, or a double ketone of oxetane.
Malonic anhydride was first synthesized in 1988 by ozonolysis of diketene.[1][2] Some derivatives, such as 3,3-dimethyl-oxetane-2,4-dione, are known.[3][4][5]
| Names | |
|---|---|
| Preferred IUPAC name Oxetane-2,4-dione | |
| Other names Malonic anhydride |
- Cotton, F. A.; Wilkinson, G. (1988) Advanced Inorganic Chemistry, 5th edn. Wiley
- ^ H. Mark Perks and Joel F. Liebman (2000). "Paradigms and Paradoxes: Aspects of the Energetics of Carboxylic Acids and Their Anhydrides". Structural Chemistry. 11(4): 265–269. doi:10.1023/A:1009270411806. ISSN 1040-0400. S2CID 92816468.
- ^ Charles L. Perrin; Arrhenius, T (1978). J. Am. Chem. SOC. volume 100, pages 5249-5251.
- ^ Ribeiro da Silva, M. A. J.; Monte, M. J. S.; Ribeiro, J. R.(1999) J. Chem.Thermodyn. 31, 1093.
- ^ Charles L. Perrin, Douglas Magde, Sylvia J. Berens, Julie Roque (1980), Raman spectrum of a malonic anhydride. (Actually, of 3,3-dimethyl-oxetane-2,4-dione.) J. Org. Chem., volume 45 issue 9, pp 1705–1706. doi:10.1021/jo01297a044.
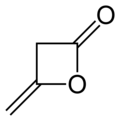
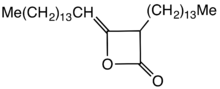
| Names | |
|---|---|
| Preferred IUPAC name 4-Methylideneoxetan-2-one | |
| Other names γ-Methylenepropiolactone |
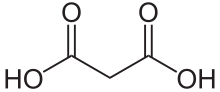
Malonic acid (IUPAC systematic name: propanedioic acid) is a dicarboxylic acidwith structure CH2(COOH)2. The ionized form of malonic acid, as well as its estersand salts, are known as malonates. For example, diethyl malonate is malonic acid's diethyl ester. The name originates from the Greek word μᾶλον (malon) meaning 'apple'.
| Names | |
|---|---|
| IUPAC name Malonic acid | |
| Preferred IUPAC name Propanedioic acid[1] | |
| Other names Methanedicarboxylic acid |
Carbon suboxide, or tricarbon dioxide, is an oxide of carbon with chemical formulaC
3O
2 or O=C=C=C=O. Its four cumulative double bonds make it a cumulene. It is one of the stable members of the series of linear oxocarbons O=Cn=O, which also includes carbon dioxide (CO2) and pentacarbon dioxide (C
5O
2). Although if carefully purified it can exist at room temperature in the dark without decomposing, it will polymerize under certain conditions.
The substance was discovered in 1873 by Benjamin Brodie by subjecting carbon monoxide to an electric current. He claimed that the product was part of a series of "oxycarbons" with formulas Cx+1Ox, namely C2O, C
3O
2, C4O3, C
5O
4, ..., and to have identified the last two;[3][4] however, only C
3O
2 is known. In 1891 Marcellin Berthelotobserved that heating pure carbon monoxide at about 550 °C created small amounts of carbon dioxide but no trace of carbon, and assumed that a carbon-rich oxide was created instead, which he named "sub-oxide". He assumed it was the same product obtained by electric discharge and proposed the formula C
2O.[5] Otto Diels later stated that the more organic names dicarbonylmethane and dioxallene were also correct.
It is commonly described as an oily liquid or gas at room temperature with an extremely noxious odor.[6]

| Names | |
|---|---|
| Preferred IUPAC name Propa-1,2-diene-1,3-dione |
https://en.wikipedia.org/wiki/Carbon_suboxide
3-Oxetanone, also called oxetan-3-one or 1,3-epoxy-2-propanone, is a chemical compound with formula C3H4O2. It is the ketone of oxetane, and an isomer of β-propiolactone.
3-Oxetanone is a liquid at room temperature, that boils at 140 °C. It is a specialty chemical,[1][2] used for research in the synthesis of other oxetanes of pharmacological interest.[3][4] Oxetan-3-one also has been the object of theoretical studies.[5][6]

| Names | |
|---|---|
| Preferred IUPAC name Oxetan-3-one | |
| Other names 1,3-Epoxy-2-propanone 1,3-Epoxypropanone 1,3-Epoxy-2-propan-2-one |

| Names | |
|---|---|
| IUPAC name 3-methyloxiran-2-one | |
| Other names α-Propiolactone 2-methyl-α-lactone |
Acetolactone or α-acetolactone is an organic compound with formula C2H2O2. It is the smallest member of the lactone family but can also be described as the epoxide of ketene. The compound was described in 1997 as a transient species in mass spectrometry experiments.[1]
Although acetolactone itself has not been isolated in bulk, the related species bis(trifluoromethyl)acetolactone ((CF3)2C2O2), which enjoys a degree of electronic stabilisation from its two trifluoromethyl groups, is known and has a half-life of 8 h at 25 °C. This compound is prepared by photolysis of bis(trifluoromethyl)malonyl peroxide.[2]

| Names | |
|---|---|
| Preferred IUPAC name Oxiranone |
https://en.wikipedia.org/wiki/Acetolactone
Oxalic anhydride or ethanedioic anhydride, also called oxiranedione, is a hypothetical organic compound, one of several isomers having the formula C2O3 that have been studied computationally. It can be viewed as the anhydride of oxalic acidor the two-fold ketone of ethylene oxide. It is an oxide of carbon (an oxocarbon).
The simple compound apparently has yet to be observed (as of 2009). In 1998, however, Paolo Strazzolini and others have claimed the synthesis of dioxane tetraketone (C4O6), which can be viewed as the cyclic dimer of oxalic anhydride.[1]
It has been conjectured to be a fleeting intermediate in the thermal decomposition of certain oxalates[2] and certain chemoluminescent reactions of oxalyl chloride.[3]

| Names | |
|---|---|
| Preferred IUPAC name Oxiranedione | |
| Other names oxalic anhydride ethanedioic anhydride |
https://en.wikipedia.org/wiki/Oxalic_anhydride
The chemical compound 1,2-dioxetanedione, or 1,2-dioxacyclobutane-3,4-dione, often called peroxyacid ester, is an unstable oxide of carbon (an oxocarbon) with formula C2O4. It can be viewed as a double ketone of 1,2-dioxetane (1,2-dioxacyclobutane), or a cyclic dimer of carbon dioxide.[1]
In ordinary conditions, it quickly decomposes to carbon dioxide (CO2) even at 180 K (−93.1 °C), but can be detected by mass spectrometry and other techniques.[2][3]
1,2-Dioxetanedione is an intermediate in the chemoluminescent reactions used in glowsticks.[4][5] The decomposition proceeds via a paramagnetic oxalate biradicalintermediate.[6]
Recently it has been found that a high-energy intermediate in one of these reactions (between oxalyl chloride and hydrogen peroxide in ethyl acetate), which is presumed to be 1,2-dioxetanedione, can accumulate in solution at room temperature (up to a few micromoles at least), provided that the activating dye and all traces of metals and other reducing agents are removed from the system, and the reactions are carried out in an inert atmosphere.[7]

| Names | |
|---|---|
| Preferred IUPAC name 1,2-Dioxetanedione | |
| Other names Peroxyacid ester |
https://en.wikipedia.org/wiki/1,2-Dioxetanedione
Category:Oxetanes
| Wikimedia Commons has media related to Oxetanes. |
Pages in category "Oxetanes"
The following 10 pages are in this category, out of 10 total. This list may not reflect recent changes (learn more).
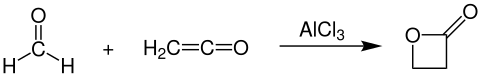
No comments:
Post a Comment