Desalination is a process that takes away mineral components from saline water. More generally, desalination refers to the removal of salts and minerals from a target substance,[1] as in soil desalination, which is an issue for agriculture. Saltwater(especially sea water) is desalinated to produce water suitable for human consumption or irrigation. The by-product of the desalination process is brine.[2] Desalination is used on many seagoing ships and submarines. Most of the modern interest in desalination is focused on cost-effective provision of fresh water for human use. Along with recycled wastewater, it is one of the few rainfall-independent water resources.[3]
Due to its energy consumption, desalinating sea water is generally more costly than fresh water from surface water or groundwater, water recycling and water conservation. However, these alternatives are not always available and depletion of reserves is a critical problem worldwide.[4][5] Desalination processes are usually driven by either thermal (in the case of distillation) or mechanical (in the case of reverse osmosis) as the primary energy types.
https://en.wikipedia.org/wiki/Desalination#Thermoionic_process
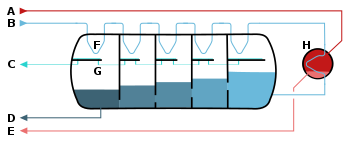
A – steam in B – seawater in C – potable water out
D – brine out (waste) E – condensate out F – heat exchange G – condensation collection (desalinated water)
H – brine heater
The pressure vessel acts as a countercurrent heat exchanger. A vacuum pump lowers the pressure in the vessel to facilitate the evaporation of the heated sea water (brine) which enters the vessel from the right side (darker shades indicate lower temperature). The steam condenses on the pipes on top of the vessel in which the fresh sea water moves from the left to the right.
Multi-stage flash distillation (MSF) is a water desalination process that distills sea water by flashing a portion of the water into steam in multiple stages of what are essentially countercurrent heat exchangers. Multi-stage flash distillation plants produce about 26% of all desalinated water in the world, but almost all of new desalination plants currently use reverse osmosis due to much lower energy consumption.[1]
Principle[edit]
The plant has a series of spaces called stages, each containing a heat exchanger and a condensate collector. The sequence has a cold end and a hot end while intermediate stages have intermediate temperatures. The stages have different pressures corresponding to the boiling points of water at the stage temperatures. After the hot end there is a container called the brine heater.
When the plant is operating in steady state, feed water at the cold inlet temperature flows, or is pumped, through the heat exchangers in the stages and warms up. When it reaches the brine heater it already has nearly the maximum temperature. In the heater, an amount of additional heat is added. After the heater, the water flows through valves back into the stages that have ever lower pressure and temperature. As it flows back through the stages the water is now called brine, to distinguish it from the inlet water. In each stage, as the brine enters, its temperature is above the boiling point at the pressure of the stage, and a small fraction of the brine water boils ("flashes") to steam thereby reducing the temperature until an equilibrium is reached. The resulting steam is a little hotter than the feed water in the heat exchanger. The steam cools and condenses against the heat exchanger tubes, thereby heating the feed water as described earlier.[2]
The total evaporation in all the stages is up to approximately 85% of the water flowing through the system, depending on the range of temperatures used. With increasing temperature there are growing difficulties of scale formation and corrosion. 110-120 °C appears to be a maximum, although scale avoidance may require temperatures below 70 °C.[3]
The feed water carries away the latent heat of the condensed steam, maintaining the low temperature of the stage. The pressure in the chamber remains constant as equal amounts of steam is formed when new warm brine enters the stage and steam is removed as it condenses on the tubes of the heat exchanger. The equilibrium is stable, because if at some point more vapor forms, the pressure increases and that reduces evaporation and increases condensation.
In the final stage the brine and the condensate has a temperature near the inlet temperature. Then the brine and condensate are pumped out from the low pressure in the stage to the ambient pressure. The brine and condensate still carry a small amount of heat that is lost from the system when they are discharged. The heat that was added in the heater makes up for this loss.
The heat added in the brine heater usually comes in the form of hot steam from an industrial process co-located with the desalination plant. The steam is allowed to condense against tubes carrying the brine (similar to the stages).
The energy that makes possible the evaporation is all present in the brine as it leaves the heater. The reason for letting the evaporation happen in multiple stages rather than a single stage at the lowest pressure and temperature, is that in a single stage, the feed water would only warm to an intermediate temperature between the inlet temperature and the heater, while much of the steam would not condense and the stage would not maintain the lowest pressure and temperature.
Such plants can operate at 23–27 kWh/m3 (appr. 90 MJ/m3) of distilled water.[4]
Because the colder salt water entering the process counterflows with the saline waste water/distilled water, relatively little heat energy leaves in the outflow—most of the heat is picked up by the colder saline water flowing toward the heater and the energy is recycled.
In addition, MSF distillation plants, especially large ones, are often paired with power plants in a cogeneration configuration. Waste heat from the power plant is used to heat the seawater, providing cooling for the power plant at the same time. This reduces the energy needed by half to two-thirds, which drastically alters the economics of the plant, since energy is by far the largest operating cost of MSF plants. Reverse osmosis, MSF distillation's main competitor, requires more pretreatment of the seawater and more maintenance, as well as energy in the form of work (electricity, mechanical power) as opposed to cheaper low-grade waste heat.[5][6]
See also[edit]
- Marine flash distillers
- Multi-effect distillation
- Multiple-effect distillation
- Reverse osmosis
- Reverse osmosis plant
- Regenerative heat exchanger
https://en.wikipedia.org/wiki/Multi-stage_flash_distillation
An evaporator, distiller or distilling apparatus is a piece of ship's equipment used to produce fresh drinking water from sea water by distillation. As fresh water is bulky, may spoil in storage, and is an essential supply for any long voyage, the ability to produce more fresh water in mid-ocean is important for any ship.
Vapour-compression distillers[edit]
Diesel-powered motorships do not use steam boilers as part of their main propulsion system and so may not have steam supplies available to drive evaporators. Some do, as they use auxiliary boilers for non-propulsion tasks such as this. Such boilers may even be heat-recovery boilers that are heated by the engine exhaust.[32]
Where no adequate steam supply is available, a vapour-compression distiller is used instead. This is driven mechanically, either electrically or by its own diesel engine.[33]
Seawater is pumped into an evaporator, where it is boiled by a heating coil. Vapour produced is then compressed, raising its temperature. This heated vapour is used to heat the evaporator coils. Condensate from the coil outlet provides the fresh water supply. To start the cycle, an electric pre-heater is used to heat the first water supply. The main energy input to the plant is in mechanically driving the compressor, not as heat energy.[33]
Both the fresh water production and the waste brine from the evaporator are led through an output cooler. This acts as a heat exchanger with the inlet seawater, pre-heating it to improve efficiency. The plant may operate at either a low pressure or slight vacuum, according to design. As the evaporator works at pressure, not under vacuum, boiling may be violent. To avoid the risk of priming and a carry over of saltwater into the vapour, the evaporator is divided by a bubble cap separator.[33]
Submarines[edit]
Vapour-compression distillers were installed on US submarines shortly before World War 2.[34] Early attempts had been made with evaporators running from diesel engine exhaust heat, but these could only be used when the submarine was running at speed on the surface. A further difficulty with submarines was the need to produce high-quality water for topping up their large storage batteries. Typical consumption on a war patrol was around 500 US gallons (1,900 litres) per day for hotel services, drinking, cooking, washing[ii] etc. and also for replenishing the diesel engine cooling system. A further 500 gallons per week was required for the batteries.[34]The standard Badger model X-1 for diesel submarines could produce 1,000 gallons per day. Tank capacity of 5,600 gallons (1,200 of which was battery water) was provided, around 10 days reserve.[34] With the appearance of nuclear submarines and their plentiful electricity supply, even larger plants could be installed. The X-1 plant was designed so that it could be operated when snorkelling, or even when completely submerged. As the ambient pressure increased when submerged, and thus the boiling point, additional heat was required in these submarine distillers, and so they were designed to run with electric heat continuously.[34]
See also[edit]
https://en.wikipedia.org/wiki/Evaporator_(marine)#Flash_distillers
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation. Dry distillation is the heating of solid materials to produce gaseous products (which may condense into liquids or solids). Dry distillation may involve chemical changes such as destructive distillation or cracking and is not discussed under this article. Distillation may result in essentially complete separation (nearly pure components), or it may be a partial separation that increases the concentration of selected components in the mixture. In either case, the process exploits differences in the relative volatility of the mixture's components. In industrial applications, distillation is a unit operation of practically universal importance, but it is a physical separation process, not a chemical reaction.
Distillation has many applications. For example:
- The distillation of fermented products produces distilled beverages with a high alcohol content, or separates other fermentation products of commercial value.
- Distillation is an effective and traditional method of desalination.
- In the petroleum industry, oil stabilization is a form of partial distillation that reduces the vapor pressure of crude oil, thereby making it safe for storage and transport as well as reducing the atmospheric emissions of volatile hydrocarbons. In midstream operations at oil refineries, fractional distillation is a major class of operation for transforming crude oil into fuels and chemical feed stocks.[2][3][4]
- Cryogenic distillation leads to the separation of air into its components – notably oxygen, nitrogen, and argon – for industrial use.
- In the chemical industry, large amounts of crude liquid products of chemical synthesis are distilled to separate them, either from other products, from impurities, or from unreacted starting materials.
An installation used for distillation, especially of distilled beverages, is a distillery. The distillation equipment itself is a still.
https://en.wikipedia.org/wiki/Distillation
Ambiguities
Waveguide, Airplane, Magnetic Landing Strip, Buoyancy, Liquid Air, Fuel, Material Properties, Ideal Gas Law, Universe Law. Space Tethers, Electric Grid, Electrodynamic Tethers, Buoys, hydrodynamics, electrodynamics, aerodynamics, statics, gravity, earth shape, aerogel, wireless, linear path, curve, sewage pipe system, roman lead pipes, water pipe system, nuclear reaction, nuclear reactor, nuclear classification, transposition, magnetosphere, earth core symmetry earth sphere/globe/etc., optics, prism, phonon, acoustics, large scale acoustic operations, geodesics, seismic waves, thermoionic emissions, pipes, pipelines, underground tunnel, channel, dams, water dam, electrodam, parabola, parabolic dish, mining, digging, oil drilling, sea pipe lines, transatlantic pipeline, trains, cars, airplane front cockpit window, etc..
Dessicant, hydrogen atmosphere concentration and hydrogen isolation capability, desalination, salt, water, ocean, oxygen, hydrogen, proton or hydrogen, protium charge particle wave, nucleon, mirror, dry air research, superfluid, dark matter universe, astronomy, universal theory, nothing on hydroelectric gas, etc..
Plasmid, plasmodium, plasmoid, plastid, plankton, prokaryote, protist, protozoa, plasma, plasmalemma, plasma membrane, cell, flagellate, cilia, cryptophyceae, etc..
protoazoa, protazoa, etc..
https://en.wikipedia.org/wiki/Desalination#Thermoionic_process
Qubit in ion-trap quantum computing[edit]
The hyperfine states of a trapped ion are commonly used for storing qubits in ion-trap quantum computing. They have the advantage of having very long lifetimes, experimentally exceeding ~10 minutes (compared to ~1 s for metastable electronic levels).
The frequency associated with the states' energy separation is in the microwave region, making it possible to drive hyperfine transitions using microwave radiation. However, at present no emitter is available that can be focused to address a particular ion from a sequence. Instead, a pair of laser pulses can be used to drive the transition, by having their frequency difference (detuning) equal to the required transition's frequency. This is essentially a stimulated Raman transition. In addition, near-field gradients have been exploited to individually address two ions separated by approximately 4.3 micrometers directly with microwave radiation.[16]
See also[edit]
https://en.wikipedia.org/wiki/Hyperfine_structure
Saturday, September 18, 2021
Saturday, September 18, 2021
Sunday, September 19, 2021
09-19-2021-0918 - Oxy-fuel welding (commonly called oxyacetylene welding, oxy welding, or gas welding in the United States) and oxy-fuel cutting
In a single-sided version, the magnetic field can create repulsion forces that push the conductor away from the stator, levitating it and carrying it along the direction of the moving magnetic field. Laithwaite called the later versions a magnetic river. These versions of the linear induction motor use a principle called transverse flux where two opposite poles are placed side by side. This permits very long poles to be used, and thus permits high speed and efficiency.[5]
https://en.wikipedia.org/wiki/Linear_induction_motor
https://en.wikipedia.org/wiki/Dihedral_angle#In_stereochemistry
https://en.wikipedia.org/wiki/Astrophysical_jet#Relativistic_jet
https://en.wikipedia.org/wiki/Electromotive_force
Sunday, September 19, 2021
09-19-2021-1434 - dihedral angle
https://en.wikipedia.org/wiki/Electrical_energy
https://en.wikipedia.org/wiki/Transducer
https://en.wikipedia.org/wiki/Electromotive_force
https://en.wikipedia.org/wiki/Chemical_energy
https://en.wikipedia.org/wiki/Mechanical_energy
https://en.wikipedia.org/wiki/Van_de_Graaff_generator
https://en.wikipedia.org/wiki/Volt#Water-flow_analogy
https://en.wikipedia.org/wiki/Geomagnetic_storm
https://en.wikipedia.org/wiki/Voltage_source
https://en.wikipedia.org/wiki/Chemical_bond
https://en.wikipedia.org/wiki/Electrolyte
https://en.wikipedia.org/wiki/Electrode
https://en.wikipedia.org/wiki/Electric_current
https://en.wikipedia.org/wiki/Galvanic_cell
https://en.wikipedia.org/wiki/Electric_potential
https://en.wikipedia.org/wiki/Scalar_field
https://en.wikipedia.org/wiki/Conjugate_variables_(thermodynamics)
https://en.wikipedia.org/wiki/Electromagnetic_induction
https://en.wikipedia.org/wiki/loop
https://en.wikipedia.org/wiki/Launch_loop
https://en.wikipedia.org/wiki/Maglev
https://en.wikipedia.org/wiki/Thermionic_emission




No comments:
Post a Comment