A "photoelectrochemical cell" is one of two distinct classes of device. The first produces electrical energy similarly to a dye-sensitized photovoltaic cell, which meets the standard definition of a photovoltaic cell. The second is a photoelectrolytic cell, that is, a device which uses light incident on a photosensitizer, semiconductor, or aqueous metal immersed in an electrolytic solution to directly cause a chemical reaction, for example to produce hydrogen via the electrolysis of water.
Both types of device are varieties of solar cell, in that a photoelectrochemical cell's function is to use the photoelectric effect (or, very similarly, the photovoltaic effect) to convert electromagnetic radiation (typically sunlight) either directly into electrical power, or into something which can itself be easily used to produce electrical power (hydrogen, for example, can be burned to create electrical power, see photohydrogen).
https://en.wikipedia.org/wiki/Photoelectrochemical_cell
Electricity generation is the process of generating electric power from sources of primary energy. For utilities in the electric power industry, it is the stage prior to its delivery (transmission, distribution, etc.) to end users or its storage (using, for example, the pumped-storage method).
Electricity is not freely available in nature, so it must be "produced" (that is, transforming other forms of energy to electricity). Production is carried out in power stations (also called "power plants"). Electricity is most often generated at a power plant by electromechanical generators, primarily driven by heat engines fueled by combustion or nuclear fission but also by other means such as the kinetic energy of flowing water and wind. Other energy sources include solar photovoltaics and geothermal power.
https://en.wikipedia.org/wiki/Electricity_generation
Shielded metal arc welding (SMAW), also known as manual metal arc welding (MMA or MMAW), flux shielded arc welding[1] or informally as stick welding, is a manual arc welding process that uses a consumable electrode covered with a flux to lay the weld.
An electric current, in the form of either alternating current or direct current from a welding power supply, is used to form an electric arc between the electrode and the metals to be joined. The workpiece and the electrode melts forming a pool of molten metal (weld pool) that cools to form a joint. As the weld is laid, the flux coating of the electrode disintegrates, giving off vapors that serve as a shielding gas and providing a layer of slag, both of which protect the weld area from atmospheric contamination.
Because of the versatility of the process and the simplicity of its equipment and operation, shielded metal arc welding is one of the world's first and most popular welding processes. It dominates other welding processes in the maintenance and repair industry, and though flux-cored arc welding is growing in popularity, SMAW continues to be used extensively in the construction of heavy steel structures and in industrial fabrication. The process is used primarily to weld iron and steels (including stainless steel) but aluminium, nickel and copperalloys can also be welded with this method.[2]
https://en.wikipedia.org/wiki/Shielded_metal_arc_welding
An electric arc, or arc discharge, is an electrical breakdown of a gas that produces a prolonged electrical discharge. The current through a normally nonconductive medium such as air produces a plasma; the plasma may produce visible light. An arc discharge is characterized by a lower voltage than a glow discharge and relies on thermionic emission of electrons from the electrodes supporting the arc. An archaic term is voltaic arc, as used in the phrase "voltaic arc lamp".
Techniques for arc suppression can be used to reduce the duration or likelihood of arc formation.
In the late 1800s, electric arc lighting was in wide use for public lighting. Some low-pressure electric arcs are used in many applications. For example, fluorescent tubes, mercury, sodium, and metal-halide lamps are used for lighting; xenon arc lampshave been used for movie projectors. Electric arcs can be utilized for manufacturing processes, such as electric arc weldingand electric arc furnaces for steel recycling.
https://en.wikipedia.org/wiki/Electric_arc
Homogeneity and heterogeneity are concepts often used in the sciences and statistics relating to the uniformity of a substance or organism. A material or image that is homogeneous is uniform in composition or character (i.e. color, shape, size, weight, height, distribution, texture, language, income, disease, temperature, radioactivity, architectural design, etc.); one that is heterogeneous is distinctly nonuniform in one of these qualities.[1][2]
https://en.wikipedia.org/wiki/Homogeneity_and_heterogeneity#Etymology_and_spelling
Symmetry (from Greek συμμετρία symmetria "agreement in dimensions, due proportion, arrangement")[1] in everyday language refers to a sense of harmonious and beautiful proportion and balance.[2][3][a] In mathematics, "symmetry" has a more precise definition, and is usually used to refer to an object that is invariant under some transformations; including translation, reflection, rotation or scaling.[4] Although these two meanings of "symmetry" can sometimes be told apart, they are intricately related, and hence are discussed together in this article.
Mathematical symmetry may be observed with respect to the passage of time; as a spatial relationship; through geometric transformations; through other kinds of functional transformations; and as an aspect of abstract objects, including theoretic models, language, and music.[5][b]
This article describes symmetry from three perspectives: in mathematics, including geometry, the most familiar type of symmetry for many people; in science and nature; and in the arts, covering architecture, art and music.
The opposite of symmetry is asymmetry, which refers to the absence or a violation of symmetry.
https://en.wikipedia.org/wiki/Symmetry
In Euclidean geometry, uniform scaling (or isotropic scaling[1]) is a linear transformation that enlarges (increases) or shrinks (diminishes) objects by a scale factor that is the same in all directions. The result of uniform scaling is similar (in the geometric sense) to the original. A scale factor of 1 is normally allowed, so that congruent shapes are also classed as similar. Uniform scaling happens, for example, when enlarging or reducing a photograph, or when creating a scale model of a building, car, airplane, etc.
More general is scaling with a separate scale factor for each axis direction. Non-uniform scaling (anisotropic scaling) is obtained when at least one of the scaling factors is different from the others; a special case is directional scaling or stretching (in one direction). Non-uniform scaling changes the shape of the object; e.g. a square may change into a rectangle, or into a parallelogram if the sides of the square are not parallel to the scaling axes (the angles between lines parallel to the axes are preserved, but not all angles). It occurs, for example, when a faraway billboard is viewed from an oblique angle, or when the shadow of a flat object falls on a surface that is not parallel to it.
When the scale factor is larger than 1, (uniform or non-uniform) scaling is sometimes also called dilation or enlargement. When the scale factor is a positive number smaller than 1, scaling is sometimes also called contraction.
In the most general sense, a scaling includes the case in which the directions of scaling are not perpendicular. It also includes the case in which one or more scale factors are equal to zero (projection), and the case of one or more negative scale factors (a directional scaling by -1 is equivalent to a reflection).
Scaling is a linear transformation, and a special case of homothetic transformation. In most cases, the homothetic transformations are non-linear transformations.
https://en.wikipedia.org/wiki/Scaling_(geometry)
A metal-halide lamp is an electrical lamp that produces light by an electric arc through a gaseous mixture of vaporized mercury and metal halides[1][2] (compounds of metals with bromine or iodine). It is a type of high-intensity discharge (HID) gas discharge lamp.[1] Developed in the 1960s, they are similar to mercury vapor lamps,[1] but contain additional metal halide compounds in the quartz arc tube, which improve the efficiency and color rendition of the light. The most common metal halide compound used is sodium iodide. Once the arc tube reaches its running temperature, the sodium dissociates from the iodine, adding orange and reds to the lamp's spectrum from the sodium D line as the metal ionizes. As a result, metal-halide lamps have high luminous efficacy of around 75–100 lumens per watt,[2] which is about twice that of mercury vapor lights and 3 to 5 times that of incandescent lights[1] and produce an intense white light. Lamp life is 6,000 to 15,000 hours.[2][3] As one of the most efficient sources of high CRI white light, metal halides as of 2005 were the fastest growing segment of the lighting industry.[1] They are used for wide area overhead lighting[2] of commercial, industrial, and public places, such as parking lots, sports arenas, factories, and retail stores,[1] as well as residential security lighting and automotive headlamps (xenon headlights).
The lamps consist of a small fused quartz or ceramic arc tube which contains the gases and the arc, enclosed inside a larger glass bulb which has a coating to filter out the ultraviolet light produced.[1][3] They operate at a pressure between 4 and 20 atmospheres, and require special fixtures to operate safely, as well as an electrical ballast. Metal atoms produce most of the light output.[1] They require a warm-up period of several minutes to reach full light output.[2]
Operation[edit]
Like other gas-discharge lamps such as the very-similar mercury-vapor lamps, metal-halide lamps produce light by ionizing a mixture of gases in an electric arc. In a metal-halide lamp, the compact arc tube contains a mixture of argon or xenon, mercury, and a variety of metal halides, such as sodium iodide and scandium iodide.[5]The particular mixture of metal halides influences the correlated color temperature and intensity (making the light more blue or red, for example). When started, the argon gas in the lamp is ionized first, which helps to maintain the arc across the two electrodes with the applied starting voltage. The heat generated by the arc and electrodes then ionizes the mercury and metal halides into a plasma, which produces an increasingly-brighter harsh white light as the temperature and pressure increases to operating conditions.
The arc-tube operates at anywhere from 5–50 atm or more[6] (70–700 psi or 500–5000 kPa) and 1000–3000 °C.[7] Like all other gas-discharge lamps, metal-halide lamps have negative resistance (with the rare exception of self-ballasted lamps with a filament), and so require a ballast to provide proper starting and operating voltages while regulating the current flow through the lamp. About 24% of the energy used by metal-halide lamps produces light (an efficacy of 65–115 lm/W),[4]making them substantially more efficient than incandescent bulbs, which typically have efficiencies in the range 2–4%.
https://en.wikipedia.org/wiki/Metal-halide_lamp
The color temperature of a light source is the temperature of an ideal black-body radiator that radiates light of a color comparable to that of the light source. Color temperature is a characteristic of visible light that has important applications in lighting, photography, videography, publishing, manufacturing, astrophysics, horticulture, and other fields. In practice, color temperature is meaningful only for light sources that do in fact correspond somewhat closely to the radiation of some black body, i.e., light in a range going from red to orange to yellow to white to blueish white; it does not make sense to speak of the color temperature of, e.g., a green or a purple light. Color temperature is conventionally expressed in kelvins, using the symbol K, a unit of measure for absolute temperature.
Color temperatures over 5000 K are called "cool colors" (bluish), while lower color temperatures (2700–3000 K) are called "warm colors" (yellowish). "Warm" in this context is an analogy to radiated heat flux of traditional incandescent lighting rather than temperature. The spectral peak of warm-colored light is closer to infrared, and most natural warm-colored light sources emit significant infrared radiation. The fact that "warm" lighting in this sense actually has a "cooler" color temperature often leads to confusion.[1]
https://en.wikipedia.org/wiki/Color_temperature#Correlated_color_temperature
A mercury-vapor lamp is a gas-discharge lamp that uses an electric arc through vaporized mercury to produce light. The arc discharge is generally confined to a small fused quartz arc tube mounted within a larger borosilicate glass bulb. The outer bulb may be clear or coated with a phosphor; in either case, the outer bulb provides thermal insulation, protection from the ultraviolet radiation the light produces, and a convenient mounting for the fused quartz arc tube.
Mercury vapor lamps are more energy efficient than incandescent and most fluorescent lights, with luminous efficacies of 35 to 65 lumens/watt.[1] Their other advantages are a long bulb lifetime in the range of 24,000 hours and a high intensity, clear white light output.[1] For these reasons, they are used for large area overhead lighting, such as in factories, warehouses, and sports arenas as well as for streetlights. Clear mercury lamps produce white light with a bluish-green tint due to mercury's combination of spectral lines.[1] This is not flattering to human skin color, so such lamps are typically not used in retail stores.[1] "Color corrected" mercury bulbs overcome this problem with a phosphor on the inside of the outer bulb that emits white light, offering better color rendition.
They operate at an internal pressure of around one atmosphere and require special fixtures, as well as an electrical ballast. They also require a warm-up period of four to seven minutes to reach full light output. Mercury vapor lamps are becoming obsolete due to the higher efficiency and better color balance of metal halide lamps.[2]
Origins[edit]
Charles Wheatstone observed the spectrum of an electric discharge in mercury vapor in 1835, and noted the ultraviolet lines in that spectrum. In 1860, John Thomas Way used arc lamps operated in a mixture of air and mercury vapor at atmospheric pressure for lighting.[3] The German physicist Leo Arons (1860–1919) studied mercury discharges in 1892 and developed a lamp based on a mercury arc.[4] In February 1896 Herbert John Dowsing and H. S. Keating of England patented a mercury vapor lamp, considered by some to be the first true mercury vapor lamp.[5]
The first mercury vapor lamp to achieve widespread success was invented in 1901 by American engineer Peter Cooper Hewitt.[6] Hewitt was issued U.S. Patent 682,692 on September 17, 1901.[7] In 1903, Hewitt created an improved version that possessed higher color qualities which eventually found widespread industrial use.[6] The ultraviolet light from mercury vapor lamps was applied to water treatment by 1910. The Hewitt lamps used a large amount of mercury. In the 1930s, improved lamps of the modern form, developed by the Osram-GEC company, General Electric company and others led to widespread use of mercury vapor lamps for general lighting.
Principle of operation[edit]
This section needs additional citations for verification.(April 2013) |
The mercury in the tube is a liquid at normal temperatures. It needs to be vaporized and ionized before the lamp can produce its full light output. To facilitate starting of the lamp, a third electrode is mounted near one of the main electrodes and connected through a resistor to the other main electrode. In addition to the mercury, the tube is filled with argon gas at low pressure. When power is applied, if there is sufficient voltage to ionize the argon, the ionized argon gas will strike a small arc between the starting electrode and the adjacent main electrode. As the ionized argon conducts, the heat from its arc vaporizes the liquid mercury; next, the voltage between the two main electrodes will ionize the mercury gas. An arc initiates between the two main electrodes and the lamp will then radiate[8] mainly in the ultraviolet, violet and blue emission lines. Continued vaporization of the liquid mercury increases the arc tube pressure to between 2 and 18 bar, depending on lamp size. The increase in pressure results in further brightening of the lamp.[9][10] The entire warm-up process takes roughly 4 to 7 minutes. Some bulbs include a thermal switch which shorts the starting electrode to the adjacent main electrode, extinguishing the starting arc once the main arc strikes.
The mercury vapor lamp is a negative resistance device. This means its resistance decreases as the current through the tube increases. So if the lamp is connected directly to a constant-voltage source like the power lines, the current through it will increase until it destroys itself. Therefore, it requires a ballast to limit the current through it. Mercury vapor lamp ballasts are similar to the ballasts used with fluorescent lamps. In fact, the first British fluorescent lamps were designed to operate from 80-watt mercury vapor ballasts. There are also self-ballasted mercury vapor lamps available. These lamps use a tungsten filament in series with the arc tube both to act as a resistive ballast and add full spectrum light to that of the arc tube. Self-ballasted mercury vapor lamps can be screwed into a standard incandescent light socket supplied with the proper voltage.
 Closeup after dark
Closeup after dark
Metal halide[edit]
A very closely related lamp design called the metal halide lamp uses various compounds in an amalgam with the mercury. Sodium iodide and scandium iodide are commonly in use. These lamps can produce much better quality light without resorting to phosphors. If they use a starting electrode, there is always a thermal shorting switch to eliminate any electrical potential between the main electrode and the starting electrode once the lamp is lit. (This electrical potential in the presence of the halides can cause the failure of the glass/metal seal). More modern metal halide systems do not use a separate starting electrode; instead, the lamp is started using high voltage pulses as with high-pressure sodium vapor lamps.
Self-ballasted lamps[edit]
Self-ballasted (SB) lamps are mercury vapor lamps with a filament inside connected in series with the arc tube that functions as an electrical ballast. This is the only kind of mercury vapor lamp that can be connected directly to the mains without an external ballast. These lamps have only the same or slightly higher efficiency than incandescent lamps of similar size, but have a longer life. They give light immediately on startup, but usually need a few minutes to restrike if power has been interrupted. Because of the light emitted by the filament, they have slightly better color rendering properties than mercury vapor lamps. Self-ballasted lamps are typically more expensive than a standard mercury vapor lamp.
Operation[edit]
When a mercury vapor lamp is first turned on, it will produce a dark blue glow because only a small amount of the mercury is ionized and the gas pressure in the arc tube is very low, so much of the light is produced in the ultraviolet mercury bands. As the main arc strikes and the gas heats up and increases in pressure, the light shifts into the visible range and the high gas pressure causes the mercury emission bands to broaden somewhat, producing a light that appears more nearly white to the human eye, although it is still not a continuous spectrum. Even at full intensity, the light from a mercury vapor lamp with no phosphors is distinctly bluish in color. The pressure in the quartz arc-tube rises to approximately one atmosphere once the bulb has reached its working temperature. If the discharge should be interrupted (e.g. by interruption of the electric supply), it is not possible for the lamp to restrike until the bulb cools enough for the pressure to fall considerably. The reason for a prolonged period of time before the lamp restrikes is because the elevated pressure, which leads to higher breakdown voltage of the gas inside (voltage needed to start an arc – Paschen's law), which is outside the capabilities of the ballast.
Color considerations[edit]
To correct the bluish tinge, many mercury vapor lamps are coated on the inside of the outer bulb with a phosphor that converts some portion of the ultraviolet emissions into red light. This helps to fill in the otherwise very-deficient red end of the electromagnetic spectrum. These lamps are generally called "color corrected" lamps. Most modern mercury vapor lamps have this coating. One of the original complaints against mercury lights was they tended to make people look like "bloodless corpses" because of the lack of light from the red end of the spectrum.[11] A common method of correcting this problem before phosphors were used was to operate the mercury lamp in conjunction with an incandescent lamp. There is also an increase in red color (e.g., due to the continuous radiation) in ultra-high-pressure mercury vapor lamps (usually greater than 200 atm.), which has found application in modern compact projection devices. When outside, coated or color corrected lamps can usually be identified by a blue "halo" around the light being given off.
Emission line spectrum[edit]
The strongest peaks of the emission line spectrum are[12][13]
| Wavelength (nm) | Name (see photoresist) | Color |
|---|---|---|
| 184.45 | ultraviolet (UVC) | |
| 253.7 | ultraviolet (UVC) | |
| 365.4 | I-line | ultraviolet (UVA) |
| 404.7 | H-line | violet |
| 435.8 | G-line | blue |
| 546.1 | green | |
| 578.2 | yellow-orange | |
| 650 | red |
In low-pressure mercury-vapor lamps only the lines at 184 nm and 254 nm are present. Fused silica is used in the manufacturing to keep the 184 nm light from being absorbed. In medium-pressure mercury-vapor lamps, the lines from 200–600 nm are present. The lamps can be constructed to emit primarily in the UV-A (around 400 nm) or UV-C (around 250 nm). High-pressure mercury-vapor lamps are commonly used for general lighting purposes. They emit primarily in the blue and green.
Ultraviolet hazards[edit]
Some mercury vapor lamps (including metal halide lamps) must contain a feature (or be installed in a fixture that contains a feature) that prevents ultraviolet radiation from escaping. Usually, the borosilicate glass outer bulb of the lamp performs this function but special care must be taken if the lamp is installed in a situation where this outer envelope can become damaged.[19] There have been documented cases of lamps being damaged in gymnasiums by balls striking the lamps, resulting in sun burns and eye inflammation from shortwave ultraviolet radiation.[20] When used in locations like gyms, the fixture should contain a strong outer guard or an outer lens to protect the lamp's outer bulb. Also, special "safety" lamps are made that will deliberately burn out if the outer glass is broken. This is usually achieved by using a thin carbon strip, which will burn up in the presence of air, to connect one of the electrodes.
Even with these methods, some UV radiation can still pass through the outer bulb of the lamp. This causes the aging process of some plastics used in the construction of luminaires to be accelerated, leaving them significantly discolored after only a few years' service. Polycarbonate suffers particularly from this problem, and it is not uncommon to see fairly new polycarbonate surfaces positioned near the lamp to have turned a dull, yellow color after only a short time.
Molecular spectroscopy[edit]
High-pressure mercury vapor (and some specially-designed metal-halide) lamps find application in molecular spectroscopy due to providing useful broadband continuum ("noise") energy at millimeter and terahertz wavelengths, owing to the high electron temperature of the arc plasma; the main UV emission line of ionized mercury (254 nm) correlates to a blackbody of T= 11,500 K. This property makes them among the very few simple, inexpensive sources available for generating such frequencies. For example, a standard 250-watt general-lighting mercury lamp produces significant output from 120 GHz to 6 THz. In addition, shorter wavelengths in the mid-infrared are emitted from the hot quartz arc-tube envelope. As with the ultraviolet output, the glass outer bulb is largely opaque at these frequencies and thus for this purpose needs to be removed (or omitted in purpose-made lamps).[citation needed]
Projection[edit]
Special ultra high-pressure mercury vapor lamps called Ultra-high-performance lamps are commonly used in digital video projectors, including DLP, 3LCD and LCoSprojectors.
https://en.wikipedia.org/wiki/Mercury-vapor_lamp
An electrical ballast is a device placed in series with a load to limit the amount of current in an electrical circuit.
A familiar and widely used example is the inductive ballast used in fluorescent lamps to limit the current through the tube, which would otherwise rise to a destructive level due to the negative differential resistance of the tube's voltage-current characteristic.
Ballasts vary greatly in complexity. They may be as simple as a resistor, inductor, or capacitor (or a combination of these) wired in series with the lamp; or as complex as the electronic ballasts used in compact fluorescent lamps (CFLs) and high-intensity discharge lamps (HID lamps).
https://en.wikipedia.org/wiki/Electrical_ballast
Borosilicate glass is a type of glass with silica and boron trioxide as the main glass-forming constituents. Borosilicate glasses are known for having very low coefficients of thermal expansion (≈3 × 10−6 K−1 at 20 °C), making them more resistant to thermal shock than any other common glass. Such glass is subjected to less thermal stress and can withstand temperature differentials without fracturing of about 165 °C (297 °F).[1] It is commonly used for the construction of reagent bottles and flasks as well as lighting, electronics and cookware.
Borosilicate glass is sold under various trade names, including Borosil, Duran, Pyrex, Supertek, Suprax, Simax, Bellco, Marinex (Brazil), BSA 60, BSC 51 (by NIPRO), Heatex, Endural, Schott, Refmex, Kimax, Gemstone Well, and MG (India).
https://en.wikipedia.org/wiki/Borosilicate_glass
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical formula of SiO2. Quartz is the second most abundant mineral in Earth's continental crust, behind feldspar.[9]
Quartz exists in two forms, the normal α-quartz and the high-temperature β-quartz, both of which are chiral. The transformation from α-quartz to β-quartz takes place abruptly at 573 °C (846 K; 1,063 °F). Since the transformation is accompanied by a significant change in volume, it can easily induce fracturing of ceramics or rocks passing through this temperature threshold.
There are many different varieties of quartz, several of which are semi-precious gemstones. Since antiquity, varieties of quartz have been the most commonly used minerals in the making of jewelry and hardstone carvings, especially in Eurasia.
Quartz is the mineral defining the value of 7 on the Mohs scale of hardness, a qualitative scratch method for determining the hardness of a material to abrasion.
https://en.wikipedia.org/wiki/Quartz
Street lighting in the United States was introduced by inventor Benjamin Franklin, who was the postmaster of Philadelphia, Pennsylvania. For this reason, many regard Philadelphia as the birthplace of street lighting in the US.
The colonial-era streetlights were lit by candles placed inside a glass vessel, which kept the candle from being blown out by wind. Franklin's design was four-sided, with four separate panes of glass, so that if one pane of glass was broken, the lamp did not need to be entirely replaced, and might not even blow out.
After the invention of gas lighting by William Murdoch in 1792, cities in Britain began to light their streets using gas. The United States followed suit shortly afterwards with the introduction of gas lighting to Pelham Street in Newport, Rhode Island, in 1803.[1] Throughout the 19th century, the use of gas lighting increased. Some locations in the US still use gas lights.
After Thomas Edison pioneered electric use, light bulbs were developed for the streetlights as well. The first city in the United States to successfully demonstrate electric lighting was Cleveland, Ohio, with twelve electric lights around the Public Square road system on 29 April 1879.[2] Charles F. Brush of Cleveland, Ohio wanted to publicly test his new invention the "Brush light" and needed a city to do so. The city council of Wabash, Indiana agreed to testing the lights and on March 31, 1880, Wabash became the "First Electrically Lighted City in the World" as a flood of light engulfed the town from four Brush lights mounted atop the courthouse. One of the original Brush lights is on display at the Wabash County Courthouse.[3] By the beginning of the 20th century, the number of fire-based streetlights was dwindling as developers were searching for safer and more effective ways to illuminate their streets. Fluorescent and incandescent lights became popular during the 1930s and 1940s, when automobile travel began to flourish. A street with lights was referred to as a white way during the early 20th century. Part of New York City's Broadwaywas nicknamed the Great White Way due to the massive number of electric lights used on theater marquees lining the street.
https://en.wikipedia.org/wiki/History_of_street_lighting_in_the_United_States
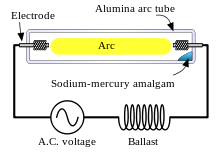
A sodium-vapor lamp is a gas-discharge lamp that uses sodium in an excited state to produce light at a characteristic wavelength near 589 nm.
Two varieties of such lamps exist: low-pressure and high-pressure. Low-pressure sodium lamps are highly efficient electrical light sources, but their yellow light restricts applications to outdoor lighting, such as street lamps, where they are widely used.[1] High-pressure sodium lamps emit a broader spectrum of light than the low-pressure lamps, but they still have poorer color renderingthan other types of lamps.[2] Low-pressure sodium lamps only give monochromatic yellow light and so inhibit color vision at night.
Development[edit]
The low-pressure sodium arc discharge lamp was first made practical around 1920 owing to the development of a type of glass that could resist the corrosive effects of sodium vapor. These operated at pressures of less than 1 Pa and produced a near monochromatic light spectrum around the sodium emission lines at 589.0 and 589.56 nanometres wavelength. The yellow light produced by these limited the range of applications to those where color vision was not required.[3]
Research into high-pressure sodium lamps occurred in both the UK and the US. Increasing the pressure of the sodium vapor broadened the sodium emission spectrum so that the light produced had more energy emitted at wavelengths above and below the 589 nm region. The quartz material used in mercury discharge lamps was corroded by high pressure sodium vapor. A laboratory demonstration of a high pressure lamp was carried out in 1959. The development by General Electric of a sintered aluminum oxide material (with magnesium oxide added to improve light transmission) was an important step in construction of a commercial lamp. The material was available in the form of tubing by 1962, but additional techniques were required to seal the tubes and add the necessary electrodes—the material could not be fused like quartz. The end caps of the arc tube would get as hot as 800 degrees C in operation, then cool to room temperature when the lamp was turned off, so the electrode terminations and arc tube seal had to tolerate repeated temperature cycles. This problem was solved by Michael Arendash[4] at the GE Nela Park plant. The first commercial high-pressure sodium lamps were available in 1965 from companies in the United States, the United Kingdom, and the Netherlands; at introduction a 400 watt lamp would produce around 100 lumens per watt.[3][5]
Single-crystal artificial sapphire tubes were also manufactured and used for HPS lamps in the early 1970s, with a slight improvement in efficacy, but production costs were higher than for polycrystalline alumina tubes.[3]
Low-pressure sodium[edit]
Low-pressure sodium (LPS) lamps have a borosilicate glass gas discharge tube (arc tube) containing solid sodium and a small amount of neon and argon gas in a Penning mixture to start the gas discharge. The discharge tube may be linear (SLI lamp)[6] or U-shaped. When the lamp is first started, it emits a dim red/pink light to warm the sodium metal; within a few minutes as the sodium metal vaporizes, the emission becomes the common bright yellow. These lamps produce a virtually monochromatic light averaging a 589.3 nm wavelength (actually two dominant spectral lines very close together at 589.0 and 589.6 nm). The colors of objects illuminated by only this narrow bandwidth are difficult to distinguish.
LPS lamps have an outer glass vacuum envelope around the inner discharge tube for thermal insulation, which improves their efficiency. Earlier LPS lamps had a detachable dewar jacket (SO lamps).[7] Lamps with a permanent vacuum envelope (SOI lamps) were developed to improve thermal insulation.[8] Further improvement was attained by coating the glass envelope with an infrared reflecting layer of indium tin oxide, resulting in SOX lamps.[9]
LPS lamps are among the most efficient electrical light sources when measured in photopic lighting conditions, producing above 100 and up to 206 lm/W.[10] This high efficiency is partly due to the light emitted being at a wavelength near the peak sensitivity of the human eye. They are used mainly for outdoor lighting (such as street lights and security lighting) where faithful color rendition is not important. Recent studies show that under typical nighttime mesopic driving conditions, whiter light can provide better results at a lower level of illumination.[11]
LPS lamps are similar to fluorescent lamps in that they are a low-intensity light source with a linear lamp shape. They do not exhibit a bright arc as do High-intensity discharge (HID) lamps; they emit a softer luminous glow, resulting in less glare. Unlike HID lamps, during a voltage dip low-pressure sodium lamps return to full brightness rapidly. LPS lamps are available with power ratings from 10 W up to 180 W; longer lamp lengths can, however, suffer design and engineering problems.
Modern LPS lamps have a service life of about 18,000 hours and do not decline in lumen output with age, though they do increase in energy consumption by about 10% towards end of life. This property contrasts with mercury vapor HID lamps, which become dimmer towards the end of life to the point of being ineffective, while consuming undiminished electrical power.
In 2017 Philips Lighting, the last manufacturer of LPS lamps, announced they were discontinuing production of the lamps due to falling demand.[12] Initially, production was due to be phased out in the course of 2020, however this date was brought forward and the last lamps were produced at the Hamilton factory in November 2019.[13]
"White" SON[edit]
A variation of the high-pressure sodium introduced in 1986, the White SON has a higher pressure than the typical HPS/SON lamp, producing a color temperature of around 2700 kelvins with a color rendering index (CRI) of about 85, greatly resembling the color of an incandescent light.[21] These lamps are often used indoors in cafes and restaurants for aesthetic effect. However, white SON lamps have higher cost, shorter service lives, and lower light efficiency, and so they cannot compete with HPS at this time.
Theory of operation[edit]
An amalgam of metallic sodium and mercury lies at the coolest part of the lamp and provides the sodium and mercury vapor that is needed to draw an arc. The temperature of the amalgam is determined to a great extent by lamp power. The higher the lamp power, the higher will be the amalgam temperature. The higher the temperature of the amalgam, the higher will be the mercury and sodium vapor pressures in the lamp and the higher will be the terminal voltage. As the temperature rises, the constant current and increasing voltage consumes increasing energy until the operating level of power is reached. For a given voltage, there are generally three modes of operation:
- The lamp is extinguished and no current flows.
- The lamp is operating with liquid amalgam in the tube.
- The lamp is operating with all amalgam evaporated.
The first and last states are stable, because the lamp resistance is weakly related to the voltage, but the second state is unstable. Any anomalous increase in current will cause an increase in power, causing an increase in amalgam temperature, which will cause a decrease in resistance, which will cause a further increase in current. This will create a runaway effect, and the lamp will jump to the high-current state (#3). Because actual lamps are not designed to handle this much power, this would result in catastrophic failure. Similarly, an anomalous drop in current will drive the lamp to extinction. It is the second state that is the desired operating state of the lamp, because a slow loss of the amalgam over time from a reservoir will have less effect on the characteristics of the lamp than a fully evaporated amalgam. The result is an average lamp life in excess of 20,000 hours.
In practical use, the lamp is powered by an AC voltage source in series with an inductive "ballast" in order to supply a nearly constant current to the lamp, rather than a constant voltage, thus assuring stable operation. The ballast is usually inductive rather than simply being resistive to minimize energy waste from resistance losses. Because the lamp effectively extinguishes at each zero-current point in the AC cycle, the inductive ballast assists in the reignition by providing a voltage spike at the zero-current point.
The light from the lamp consists of atomic emission lines of mercury and sodium, but is dominated by the sodium D-line emission. This line is extremely pressure (resonance) broadened and is also self-reversed because of absorption in the cooler outer layers of the arc, giving the lamp its improved color rendering characteristics. In addition, the red wing of the D-line emission is further pressure broadened by the Van der Waals forces from the mercury atoms in the arc.
End of life[edit]
At end of life, high-pressure sodium (HPS) lamps exhibit a phenomenon known as cycling, caused by a loss of sodium in the arc. Sodium is a highly reactive element and is lost in a reaction with the aluminum oxide of the arc tube. The products are sodium oxide and aluminum:
- 6 Na + Al2O3 → 3 Na2O + 2 Al
As a result, these lamps can be started at a relatively low voltage, but, as they heat up during operation, the internal gas pressure within the arc tube rises, and more and more voltage is required to maintain the arc discharge. As a lamp gets older, the maintaining voltage for the arc eventually rises to exceed the maximum voltage output by the electrical ballast. As the lamp heats to this point, the arc fails, and the lamp goes out. Eventually, with the arc extinguished, the lamp cools down again, the gas pressure in the arc tube is reduced, and the ballast can once again cause the arc to strike. The effect of this is that the lamp glows for a while and then goes out, typically starting at a pure or bluish white then moving to a red-orange before going out.
More sophisticated ballast designs detect cycling and give up attempting to start the lamp after a few cycles, as the repeated high-voltage ignitions needed to restart the arc reduce the lifetime of the ballast. If power is removed and reapplied, the ballast will make a new series of startup attempts.
LPS lamp failure does not result in cycling; rather, the lamp will simply not strike or will maintain the dull red glow of the start-up phase. In another failure mode, a tiny puncture of the arc tube leaks some of the sodium vapor into the outer vacuum bulb. The sodium condenses and creates a mirror on the outer glass, partially obscuring the arc tube. The lamp often continues operating normally, but much of the light generated is obscured by the sodium coating, providing no illumination.
https://en.wikipedia.org/wiki/Sodium-vapor_lamp
The sulfur lamp (also sulphur lamp) is a highly efficient full-spectrum electrodeless lighting system whose light is generated by sulfur plasma that has been excitedby microwave radiation. They are a particular type of plasma lamp, and one of the most modern. The technology was developed in the early 1990s, but, although it appeared initially to be very promising, sulfur lighting was a commercial failure by the late 1990s. Since 2005, lamps are again being manufactured for commercial use.
https://en.wikipedia.org/wiki/Sulfur_lamp
















No comments:
Post a Comment