Tight junctions, also known as occluding junctions or zonulae occludentes(singular, zonula occludens) are multiprotein junctional complexes whose general function is to prevent leakage of transported solutes and water and seals the paracellular pathway. Tight junctions may also serve as leaky pathways by forming selective channels for small cations, anions, or water. Tight junctions are present mostly in vertebrates (with the exception of Tunicates[1]). The corresponding junctions that occur in invertebrates are septate junctions.

Transmembrane proteins:
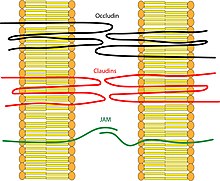
- Occludin was the first integral membrane protein to be identified. It has a molecular weight of ~60kDa. It consists of four transmembrane domains and both the N-terminus and the C-terminus of the protein are intracellular. It forms two extracellular loops and one intracellular loop. These loops help regulate paracellular permeability.[4] Occludin also plays a key role in cellular structure and barrier function.[5]
- Claudins were discovered after occludin and are a family of 24 different mammalian proteins.[6] They have a molecular weight of ~20kDa. They have a structure similar to that of occludin in that they have four transmembrane domains and similar loop structure. They are understood to be the backbone of tight junctions and play a significant role in the tight junction's ability to seal the paracellular space.[7] Different claudins are found in different locations throughout the human body.
- Junction Adhesion Molecules (JAM) are part of the immunoglobulin superfamily. They have a molecular weight of ~40kDa. Their structure differs from that of the other integral membrane proteins in that they only have one transmembrane domain instead of four. It helps to regulate the paracellular pathway function of tight junctions and is also involved in helping to maintain cell polarity.[8]
- Angulins were discovered in 2011 by visual screening of proteins which localize at tricellular tight junctions.[9] There are three members of angulins, Angulin-1/LSR, Angulin-2/ILDR1, and Angulin-3/ILDR2. Similar to JAMs, angulins are single-transmembrane proteins. All angulins have one immunoglobulin-like domain in the extracellular region and one PDZ-binding motif at the carboxy-terminus. They are responsible for establishment of tricellular tight junctions and regulate the paracellular barrier function.[10]
Functions[edit]
They perform vital functions:[11]
- They hold cells together.
- Barrier function, which can be further subdivided into protective barriers and functional barriers serving purposes such as material transport and maintenance of osmotic balance:
- Tight junctions help to maintain the polarity of cells by preventing the lateral diffusion of integral membrane proteins between the apical and lateral/basal surfaces, allowing the specialized functions of each surface (for example receptor-mediated endocytosis at the apical surface and exocytosis at the basolateral surface) to be preserved. This aims to preserve the transcellular transport.
- Tight junctions prevent the passage of molecules and ions through the space between plasma membranes of adjacent cells, so materials must actually enter the cells (by diffusion or active transport) in order to pass through the tissue. Investigation using freeze-fracture methods in electron microscopy is ideal for revealing the lateral extent of tight junctions in cell membranes and has been useful in showing how tight junctions are formed.[12] The constrained intracellular pathway exacted by the tight junction barrier system allows precise control over which substances can pass through a particular tissue. (Tight junctions play this role in maintaining the blood–brain barrier.) At the present time, it is still unclear whether the control is active or passive and how these pathways are formed. In one study for paracellular transport across the tight junction in kidney proximal tubule, a dual pathway model is proposed: large slit breaks formed by infrequent discontinuities in the TJ complex and numerous small circular pores.[13]
In human physiology there are two main types of epithelia using distinct types of barrier mechanism. Epidermal structures such as skin form a barrier from many layers of keratinized squamous cells. Internal epithelia on the other hand more often rely on tight junctions for their barrier function. This kind of barrier is mostly formed by only one or two layers of cells. It was long unclear whether tight cell junctions also play any role in the barrier function of the skin and similar external epithelia but recent research suggests that this is indeed the case.[14]
Classification[edit]
Epithelia are classed as "tight" or "leaky", depending on the ability of the tight junctions to prevent water and solute movement:[15]
- Tight epithelia have tight junctions that prevent most movement between cells. Examples of tight epithelia include the distal convoluted tubule, the collecting duct of the nephron in the kidney, and the bile ducts ramifying through liver tissue. Other examples are the blood-brain barrier and the blood cerebrospinal fluid barrier
- Leaky epithelia do not have these tight junctions, or have less complex tight junctions. For instance, the tight junction in the kidney proximal tubule, a very leaky epithelium, has only two to three junctional strands, and these strands exhibit infrequent large slit breaks.
https://en.wikipedia.org/wiki/Tight_junction
https://en.wikipedia.org/wiki/Tight_junction
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane and can either penetrate the membrane (transmembrane) or associate with one or the other side of a membrane (integral monotopic). Peripheral membrane proteins are transiently associated with the cell membrane.
Membrane proteins are common, and medically important—about a third of all human proteins are membrane proteins, and these are targets for more than half of all drugs.[1] Nonetheless, compared to other classes of proteins, determining membrane protein structures remains a challenge in large part due to the difficulty in establishing experimental conditions that can preserve the correct conformation of the protein in isolation from its native environment.
https://en.wikipedia.org/wiki/Membrane_protein
Zonulin (haptoglobin 2 precursor)[1] is a protein that modulates the permeability of tight junctions between cells of the wall of the digestive tract.[2] It was discovered in 2000 by Alessio Fasano and his team at the University of Maryland School of Medicine. As the mammalian analogue of zonula occludens toxin, secreted by cholera pathogen Vibrio cholerae, zonulin has been implicated in the pathogenesis of coeliac disease and diabetes mellitus type 1.[3] Type 2 diabetic patients have shown increased zonulin.[4]
Gliadin (glycoprotein present in wheat) activates zonulin signaling irrespective of the genetic expression of autoimmunity, leading to increased intestinal permeability to macromolecules.[3][5]
Zonula occludens toxin is being studied as an adjuvant to improve absorption of drugs and vaccines.[6] In 2014 a zonulin receptor antagonist, larazotide acetate (formerly known as AT-1001), completed a phase 2b clinical trial.[7][8]
https://en.wikipedia.org/wiki/Zonulin
Cadherins (named for "calcium-dependent adhesion") are a type of cell adhesion molecule (CAM) that are important in the formation of adherens junctions to bind cells with each other.[1] Cadherins are a class of type-1 transmembrane proteins, and they are dependent on calcium (Ca2+) ions to function, hence their name. Cell-cell adhesion is mediated by extracellular cadherin domains, whereas the intracellular cytoplasmic tail associates with numerous adaptors and signaling proteins, collectively referred as the cadherin adhesome.
The cadherin family is essential in maintaining the cell to cell contact between each other and regulating for cytoskeletal complexes. The cadherin superfamily includes cadherins, protocadherins, desmogleins, desmocollins, and more.[2][3] In structure, they share cadherin repeats, which are the extracellular Ca2+-binding domains. There are multiple classes of cadherin molecules, each designated with a prefix (in general, noting the types of tissue with which it is associated). Classical cadherins maintain the tone of tissues by forming a homodimer while desmosomes are the heterodimers.[4]The intracellular portion of classical cadherins has regulatory proteins that helps the cadherins joining the actin cytoskeleton. Although classical cadherins take a role in cell layer formation and structure formation, desmosomal cadherins focus on resisting cell damage. Desmosomal cadherins are responsible to maintain the function of desmosomes that is to overturn the mechanical stress of the tissues. Similar to classical cadherins, desmosomal cadherins has a single transmembrane domain, five EC repeats, and an intracellular domain. Two types of desmosomal cadherins exist, and they are called desmogleins and desmocollins that contains an intracellular anchor and cadherin like sequence (ICS). The adaptor proteins that associate with desmosomal cadherins are plakoglobin (related to -catenin), plakophilins (p120 catenin subfamily), and desmoplakins. The major function of desmoplakins to bind to intermediate filament thorough interaction with plakoglobin that attaches to ICS of desmogleins and desmocollins and plakophilins. A[4]typical cadherins are different from other types of cadherins and consist of one or more extracellular repeat domains. The components that build an atypical cadherin are flamingo (seven pass transmembrane) and Dcad102F-like cadherins. Their job is to take part in signaling pathway instead of performing cell-cell adhesion.
It has been observed that cells containing a specific cadherin subtype tend to cluster together to the exclusion of other types, both in cell culture and during development.[5] For example, cells containing N-cadherin tend to cluster with other N-cadherin-expressing cells. However, it has been noted that the mixing speed in the cell culture experiments can have an effect on the extent of homotypic specificity.[6] In addition, several groups have observed heterotypic binding affinity (i.e., binding of different types of cadherin together) in various assays.[7][8] One current model proposes that cells distinguish cadherin subtypes based on kinetic specificity rather than thermodynamic specificity, as different types of cadherin homotypic bonds have different lifetimes.[9]
https://en.wikipedia.org/wiki/Cadherin
Tight junction protein are proteins that are involved in the formation and functioning of tight junctions; "Tight junction protein" may refer to:
- TJP1, Tight junction protein 1 (ZO-1)
- TJP2, Tight junction protein 2 (ZO-2)
- TJP3, Tight junction protein 3 (ZO-3)
See also[edit]
Adherens junctions (or zonula adherens, intermediate junction, or "belt desmosome"[1]) are protein complexes that occur at cell–cell junctions, cell–matrix junctions in epithelial and endothelial tissues,[2]usually more basal than tight junctions. An adherens junction is defined as a cell junction whose cytoplasmic face is linked to the actincytoskeleton. They can appear as bands encircling the cell (zonula adherens) or as spots of attachment to the extracellular matrix (focal adhesion). Adherens junctions uniquely disassemble in uterine epithelial cells to allow the blastocyst to penetrate between epithelial cells.[3]
A similar cell junction in non-epithelial, non-endothelial cells is the fascia adherens. It is structurally the same, but appears in ribbonlike patterns that do not completely encircle the cells. One example is in cardiomyocytes.
https://en.wikipedia.org/wiki/Adherens_junction
A desmosome (/ˈdɛzməˌsoʊm/;[1][2] "binding body"), also known as a macula adherens (plural: maculae adherentes) (Latin for adhering spot), is a cell structure specialized for cell-to-cell adhesion. A type of junctional complex, they are localized spot-like adhesions randomly arranged on the lateral sides of plasma membranes. Desmosomes are one of the stronger cell-to-cell adhesion types and are found in tissue that experience intense mechanical stress, such as cardiac muscle tissue, bladder tissue, gastrointestinal mucosa, and epithelia.[3]
https://en.wikipedia.org/wiki/Desmosome
Cell adhesion molecules (CAMs) are a subset of cell adhesion proteins located on the cell surface[1] involved in binding with other cells or with the extracellular matrix (ECM) in the process called cell adhesion. In essence, cell adhesion molecules help cells stick to each other and to their surroundings. Cell adhesion is a crucial component in maintaining tissue structure and function. In fully developed animals, these molecules play an integral role in creating force and movement and consequently ensure that organs are able to execute their functions. In addition to serving as "molecular glue", cell adhesion is important in affecting cellular mechanisms of growth, contact inhibition, and apoptosis. Oftentimes aberrant expression of CAMs will result in pathologies ranging from frostbite to cancer.[2]
Combined with cell junctions and ECM, CAMs help hold animal cells together.
https://en.wikipedia.org/wiki/Cell_adhesion_molecule
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential,[1] shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells.[2][3] Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters.[4]
The study of ion channels often involves biophysics, electrophysiology, and pharmacology, while using techniques including voltage clamp, patch clamp, immunohistochemistry, X-ray crystallography, fluoroscopy, and RT-PCR. Their classification as molecules is referred to as channelomics.
https://en.wikipedia.org/wiki/Ion_channel
In biology, a connexon, also known as a connexin hemichannel, is an assembly of six proteins called connexins that form the pore for a gap junction between the cytoplasm of two adjacent cells. This channel allows for bidirectional flow of ions and signaling molecules.[1] The connexon is the hemichannel supplied by a cell on one side of the junction; two connexons from opposing cells normally come together to form the complete intercellular gap junction channel. However, in some cells, the hemichannel itself is active as a conduit between the cytoplasm and the extracellular space, allowing the transference of ions and small molecules lower than 1-2 KDa. Little is known about this function of connexons besides the new evidence suggesting their key role in intracellular signaling.[2]
Connexons made of the same type of connexins are considered homomeric, while connexons made of differing types of connexins are heteromeric.[3]
https://en.wikipedia.org/wiki/Connexon
Gap junctions are specialized intercellular connections between a multitude of animal cell-types.[1][2][3] They directly connect the cytoplasm of two cells, which allows various molecules, ions and electrical impulses to directly pass through a regulated gate between cells.[4][5]
One gap junction channel is composed of two protein heximers (or hemichannels) called connexons in vertebrates and innexons in invertebrates. The hemichannel pair connect across the intercellular space bridging the gap between two cells.[4][5][6] Gap junctions are analogous to the plasmodesmata that join plant cells.[7]
Gap junctions occur in virtually all tissues of the body, with the exception of adult fully developed skeletal muscle and mobile cell types such as sperm or erythrocytes. Gap junctions are not found in simpler organisms such as sponges and slime molds.
A gap junction may also be called a nexus or macula communicans. While an ephapse has some similarities to a gap junction, by modern definition the two are different.
https://en.wikipedia.org/wiki/Gap_junction
The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including bacteria and archaea.[1]It extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. In eukaryotes, it is composed of three main components, microfilaments, intermediate filaments and microtubules, and these are all capable of rapid growth or disassembly dependent on the cell's requirements.[2]
A multitude of functions can be performed by the cytoskeleton. Its primary function is to give the cell its shape and mechanical resistance to deformation, and through association with extracellular connective tissue and other cells it stabilizes entire tissues.[3][4] The cytoskeleton can also contract, thereby deforming the cell and the cell's environment and allowing cells to migrate.[5]Moreover, it is involved in many cell signaling pathways and in the uptake of extracellular material (endocytosis),[6] the segregation of chromosomes during cellular division,[3] the cytokinesis stage of cell division,[7] as scaffolding to organize the contents of the cell in space[5] and in intracellular transport (for example, the movement of vesicles and organelles within the cell)[3] and can be a template for the construction of a cell wall.[3] Furthermore, it can form specialized structures, such as flagella, cilia, lamellipodia and podosomes. The structure, function and dynamic behavior of the cytoskeleton can be very different, depending on organism and cell type.[3][7] Even within one cell, the cytoskeleton can change through association with other proteins and the previous history of the network.[5]
A large-scale example of an action performed by the cytoskeleton is muscle contraction. This is carried out by groups of highly specialized cells working together. A main component in the cytoskeleton that helps show the true function of this muscle contraction is the microfilament. Microfilaments are composed of the most abundant cellular protein known as actin.[8] During contraction of a muscle, within each muscle cell, myosin molecular motors collectively exert forces on parallel actin filaments. Muscle contraction starts from nerve impulses which then causes increased amounts of calcium to be released from the sarcoplasmic reticulum. Increases in calcium in the cytosol allows muscle contraction to begin with the help of two proteins, tropomyosinand troponin.[8] Tropomyosin inhibits the interaction between actin and myosin, while troponin senses the increase in calcium and releases the inhibition.[9] This action contracts the muscle cell, and through the synchronous process in many muscle cells, the entire muscle.
https://en.wikipedia.org/wiki/Cytoskeleton
Desmoplakin is a protein in humans that is encoded by the DSPgene.[5][6][7] Desmoplakin is a critical component of desmosome structures in cardiac muscle and epidermal cells, which function to maintain the structural integrity at adjacent cell contacts. In cardiac muscle, desmoplakin is localized to intercalated discs which mechanically couple cardiac cells to function in a coordinated syncytial structure. Mutations in desmoplakin have been shown to play a role in dilated cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, striate palmoplantar keratoderma, Carvajal syndrome and paraneoplastic pemphigus.
https://en.wikipedia.org/wiki/Desmoplakin
Plakoglobin, also known as junction plakoglobin or gamma-catenin, is a protein that in humans is encoded by the JUP gene.[5] Plakoglobin is a member of the catenin protein family and homologous to β-catenin. Plakoglobin is a cytoplasmic component of desmosomes and adherens junctions structures located within intercalated discs of cardiac muscle that function to anchor sarcomeres and join adjacent cells in cardiac muscle. Mutations in plakoglobin are associated with arrhythmogenic right ventricular dysplasia.
https://en.wikipedia.org/wiki/Plakoglobin
Tonofibrils are cytoplasmic protein structures in epithelial tissues that converge at desmosomes and hemidesmosomes.[1] They consist of fine fibrils in epithelial cells that are anchored to the cytoskeleton.[2] They were discovered by Rudolf Heidenhain, and first described in detail by Louis-Antoine Ranvier in 1897.[3]
Composition[edit]
Tonofilaments are keratin intermediate filaments that makes up tonofibrils in the epithelial tissue. In epithelial cells, tonofilaments loop through desmosomes. Electron microscopy has advanced now to illustrate the Tonofilaments more clearly.[1]
The protein filaggrin is believed to have an important role in holding them together as tonofibrils. This protein is known to interact with intermediate filaments, specifically keratins. It is synthesized as a giant Precursor protein, profilaggrin (>400 kDA in humans). When the filaggrin binds to keratin intermediate filaments, it causes aggregation in macrofibrils.[2]
https://en.wikipedia.org/wiki/Tonofibril
MARVEL domain-containing protein 2 is a protein that in humans is encoded by the MARVELD2 gene.[5][6]
https://en.wikipedia.org/wiki/MARVELD2
The basal lamina is a layer of extracellular matrix secreted by the epithelial cells, on which the epithelium sits. It is often incorrectly referred to as the basement membrane, though it does constitute a portion of the basement membrane. The basal lamina is visible only with the electron microscope, where it appears as an electron-dense layer that is 20–100 nm thick (with some exceptions that are thicker, such as basal lamina in lung alveoli and renal glomeruli).
https://en.wikipedia.org/wiki/Basal_lamina
Hemidesmosomes are very small stud-like structures found in keratinocytes of the epidermis of skin that attach to the extracellular matrix. They are similar in form to desmosomes when visualized by electron microscopy, however, desmosomes attach to adjacent cells. Hemidesmosomes are also comparable to focal adhesions, as they both attach cells to the extracellular matrix. Instead of desmogleins and desmocollinsin the extracellular space, hemidesmosomes utilize integrins. Hemidesmosomes are found in epithelial cells connecting the basal epithelial cells to the lamina lucida, which is part of the basal lamina.[2] Hemidesmosomes are also involved in signaling pathways, such as keratinocyte migration or carcinoma cell intrusion.[3]
https://en.wikipedia.org/wiki/Hemidesmosome
In cell biology, focal adhesions (also cell–matrix adhesions or FAs) are large macromolecular assemblies through which mechanical force and regulatory signals are transmitted between the extracellular matrix (ECM) and an interacting cell. More precisely, focal adhesions are the sub-cellular structures that mediate the regulatory effects (i.e., signaling events) of a cell in response to ECM adhesion.[1]
Focal adhesions serve as the mechanical linkages to the ECM, and as a biochemical signaling hub to concentrate and direct numerous signaling proteins at sites of integrin binding and clustering.
https://en.wikipedia.org/wiki/Focal_adhesion
The costamere is a structural-functional component of striated muscle cells[1] which connects the sarcomere of the muscle to the cell membrane (i.e. the sarcolemma).[2]
Costameres are sub-sarcolemmal protein assemblies circumferentially aligned in register with the Z-disk of peripheral myofibrils.[3][4][5] They physically couple force-generating sarcomeres with the sarcolemma in striated muscle cells and are thus considered one of several "Achilles' heels" of skeletal muscle, a critical component of striated muscle morphology which, when compromised, is thought to directly contribute to the development of several distinct myopathies.[6]
The dystrophin-associated protein complex (DAG), also referred to as the dystrophin-associated glycoprotein complex (DGC),[2] contains various integral and peripheral membrane proteins such as dystroglycans and sarcoglycans, which are thought to be responsible for linking the internal cytoskeletal system of individual myofibers to structural proteins within the extracellular matrix (such as collagen and laminin). Therefore, it is one of the features of the sarcolemma which helps to couple the sarcomere to the extracellular connective tissue as some experiments have shown.[7]Desmin protein may also bind to the DAG complex, and regions of it are known to be involved in signaling.
https://en.wikipedia.org/wiki/Costamere
The cilium (from Latin 'eyelash';[1] the plural is cilia) is an organelle found on eukaryotic cells in the shape of a slender protuberance that projects from the much larger cell body.[2]
There are two types of cilia: motile and non-motile cilia. Non-motile cilia are also called primary cilia which serve as sensory organelles. Most mammalian cell types possess a single non-motile, primary cilium, which functions as a cellular antenna.[3][4] Exceptions include olfactory neurons which possess several non-motile cilia and cells of the transient embryonic node, which possess singular motile cilia known as nodal cilia, critical for the establishment of left to right body asymmetry.[5]
In eukaryotes, motile cilia and flagella (together known as undulipodia) are structurally similar, although distinctions are sometimes made according to function or length.[6][7] Immotile cilia (called primary cilia) communicate signals from the environment or from other cells.[8][9]
https://en.wikipedia.org/wiki/Cilium
Microvilli (singular: microvillus) are microscopic cellular membrane protrusions that increase the surface area for diffusion and minimize any increase in volume,[1] and are involved in a wide variety of functions, including absorption, secretion, cellular adhesion, and mechanotransduction.
https://en.wikipedia.org/wiki/Microvillus
Stereocilia (or stereovilli) are non-motile apical modifications of the cell. They are distinct from cilia and microvilli, but closely related to the latter.
In structure, they are much longer and thicker than typical microvilli, form single "finger-like" projections that may be branched, and have more of the characteristics of the cellular membrane proper. Like microvilli, they contain actin filaments and lack an axoneme, distinguishing them from cilia.
They are found in three regions of the body:
- the ductus deferens
- the epididymis (see stereocilia (epididymis) for more details). Some sources consider epididymal stereocilia to be a variant of microvilli,[1] rather than their own distinct type of structure.
- the sensory (hair) cells of the inner ear (see stereocilia (inner ear) for more details)
Stereocilin is a protein that in humans is encoded by the STRCgene.[5][6][7]
This gene encodes a protein that is associated with the hair bundle of the sensory hair cells in the inner ear. The hair bundle is composed of stiff microvilli called stereocilia and is involved with mechanoreception of sound waves. This gene is part of a tandem duplication on chromosome 15; the second copy is a pseudogene. Mutations in this gene cause autosomal recessive non-syndromic deafness.[7]
https://en.wikipedia.org/wiki/STRC
Occludin is an enzyme (EC 1.6) that oxidizes NADH.[5] It was first identified in epithelial cells as a 65 kDa integral plasma-membrane protein localized at the tight junctions.[6] Together with Claudins, and zonula occludens-1 (ZO-1), occludin has been considered a staple of tight junctions, and although it was shown to regulate the formation, maintenance, and function of tight junctions, its precise mechanism of action remained elusive and most of its actions were initially attributed to conformational changes following selective phosphorylation,[7] and its redox-sensitive dimerization.[8][9] However, mounting evidence demonstrated that occludin is not only present in epithelial/endothelial cells, but is also expressed in large quantities in cells that do not have tight junctions but have very active metabolism: pericytes,[5] neurons and astrocytes,[10] oligodendrocytes,[11] dendritic cells,[12]monocytes/macrophages[13] lymphocytes,[14] and myocardium.[15] Recent work, using molecular modeling, supported by biochemical and live-cell experiments in human cells demonstrated that occludin is a NADH oxidase that influences critical aspects of cell metabolism like glucose uptake, ATP production and gene expression.[16] Furthermore, manipulation of occludin content in human cells is capable of influencing the expression of glucose transporters,[16] and the activation of transcription factors like NFkB, and histone deacetylases like sirtuins, which proved capable of diminishing HIV replication rates in infected human macrophages under laboratory conditions.[5]
https://en.wikipedia.org/wiki/Occludin
Claudins are a family of proteins which, along with occludin, are the most important components of the tight junctions (zonulae occludentes).[1][2] Tight junctions establish the paracellular barrier that controls the flow of molecules in the intercellular space between the cells of an epithelium.[1][3] They have four transmembrane domains, with the N-terminus and the C-terminus in the cytoplasm.Claudins are a family of proteins which, along with occludin, are the most important components of the tight junctions (zonulae occludentes).[1][2] Tight junctions establish the paracellular barrier that controls the flow of molecules in the intercellular space between the cells of an epithelium.[1][3] They have four transmembrane domains, with the N-terminus and the C-terminus in the cytoplasm.
https://en.wikipedia.org/wiki/Claudin
Above.


No comments:
Post a Comment