Bisphosphonate
Not to be confused with Biphosphate (disambiguation).
Bisphosphonates are a class of drugs that prevent the loss of bone density, used to treat osteoporosis and similar diseases. They are the most commonly prescribed drugs used to treat osteoporosis.[1] They are called bisphosphonates because they have two phosphonate(PO(OH)
2) groups. They are thus also called diphosphonates (bis- or di- + phosphonate).
Evidence shows that they reduce the risk of fracture in post-menopausal women with osteoporosis.[2][3][4][5][6]
Bone tissue undergoes constant remodeling and is kept in balance (homeostasis) by osteoblasts creating bone and osteoclasts destroying bone. Bisphosphonates inhibit the digestion of bone by encouraging osteoclasts to undergo apoptosis, or cell death, thereby slowing bone loss.[7]
The uses of bisphosphonates include the prevention and treatment of osteoporosis, Paget's disease of bone, bone metastasis (with or without hypercalcemia), multiple myeloma, primary hyperparathyroidism, osteogenesis imperfecta, fibrous dysplasia, and other conditions that exhibit bone fragility.
Bisphosphonates are used to treat osteoporosis, osteitis deformans (Paget's disease of the bone), bone metastasis (with or without hypercalcaemia), multiple myeloma, and other conditions involving fragile, breakable bone.
In osteoporosis and Paget's, the most popular first-line bisphosphonate drugs are alendronate and risedronate. If these are ineffective or if the person develops digestive tract problems, intravenous pamidronate may be used. Strontium ranelate or teriparatide are used for refractory disease. The use of strontium ranelate is restricted because of increased risk of venous thromboembolism, pulmonary embolism and serious cardiovascular disorders, including myocardial infarction.[8] In postmenopausal women, the selective estrogen receptor modulator raloxifene is occasionally administered instead of bisphosphonates. Bisphosphonates are beneficial in reducing the risk of vertebral fracture in steroid induced osteoporosis.[9]
Non-nitrogenous[edit]
The non-nitrogenous bisphosphonates (diphosphonates) are metabolised in the cell to compounds that replace the terminal pyrophosphate moiety of ATP, forming a non-functional molecule that competes with adenosine triphosphate (ATP) in the cellular energy metabolism. The osteoclast initiates apoptosis and dies, leading to an overall decrease in the breakdown of bone. This type of bisphosphonate has overall more negative effects than the nitrogen containing group, and is prescribed far less often.[37]
Nitrogenous
Nitrogenous bisphosphonates act on bone metabolism by binding and blocking the enzyme farnesyl diphosphate synthase (FPPS) in the HMG-CoA reductase pathway (also known as the mevalonate pathway).[38]
Bisphosphonates that contain isoprene chains at the R1 or R2 position can impart specificity for inhibition of GGPS1.[39]
Disruption of the HMG CoA-reductase pathway at the level of FPPS prevents the formation of two metabolites (farnesol and geranylgeraniol) that are essential for connecting some small proteins to the cell membrane. This phenomenon is known as prenylation, and is important for proper sub-cellular protein trafficking (see "lipid-anchored protein" for the principles of this phenomenon).[40]
While inhibition of protein prenylation may affect many proteins found in an osteoclast, disruption to the lipid modification of Ras, Rho, Rac proteins has been speculated to underlie the effects of bisphosphonates. These proteins can affect both osteoclastogenesis, cell survival, and cytoskeletal dynamics. In particular, the cytoskeleton is vital for maintaining the "ruffled border" that is required for contact between a resorbing osteoclast and a bone surface.
Statins are another class of drugs that inhibit the HMG-CoA reductase pathway. Unlike bisphosphonates, statins do not bind to bone surfaces with high affinity, and thus are not specific for bone. Nevertheless, some studies have reported a decreased rate of fracture(an indicator of osteoporosis) and/or an increased bone mineral density in statin users. The overall efficacy of statins in the treatment of osteoporosis remains controversial.[41]
The original bisphosphonates (first generation) were simple molecules with small groups of single atoms or alkyl chains in position R1 and R2. They only had a rather weak inhibiting effect on bone resorption. The inclusion of an amino group marked the beginning of the second generation of bisphosphonates with higher potency. The first was pamidronate and similar analogues followed where the position of the nitrogen in the side chain was the key to a more potent drug. Later it became apparent that the nitrogen does not necessarily have to be connected to an alkyl chain but instead using a heterocyclic group. A few such drugs have been developed and placed on the market where zoledronate is the most notable one. Minodronic acid is even more potent and has been placed on the market in Japan. Their potency is such that it is effective even in picomolar concentration.[3]
Further development has not resulted in the placing on the market of compounds in equal potency. Arylalkyl substitutes of pamidronate are among the most recent bisphosphonates to be used clinically where the hydroxyl group in position R2 has been omitted to ensure stability.[1]
Recent research in this area has opened up an opportunity to develop new bisphosphonate drug therapies.
Bisphosphonates with a more lipophilic character have been developed and have shown potential as a tumor suppressant. They operate by a slightly different mechanism in which they not only inhibit the key enzyme farnesyl pyrophosphate synthase (FPPS) of the mevalonate pathway but also geranylgeranyl pyrophosphate synthase (GGPS), an enzyme also located in the mevalonate pathway. They do not have the same affinity for the bone minerals.[4]
GGPS has since been successfully inhibited by a novel bisphosphonate compound with a triazole group within R2 and a methyl group in R1. This may become useful in therapies against malignancies like multiple myeloma.[5]
In 2018, a dendritic bisphosphonate was introduced containing three bisphosphonate units. It has shown potential for bone specific delivery of large therapeutic molecules by taking advantage of the high affinity of bisphosphonates to the bone minerals [6]
Mechanism of action[edit]
The mechanism of action of the bisphosphonates (BP's) has evolved as new generations of drugs have been developed. The function of the first generation bisphosphonates differs from the more recent nitrogen containing BP's but both are apparently internalised by endocytosis of a membrane-bound vesicle where the drug is most likely in a complex with Ca2+ ions. This does not concern other cells in the bone as this takes place by a selective uptake of osteoclasts.[3]
The common function which applies to all bisphosphonate drugs is a physicochemical interaction with the bone mineral to prevent the physical resorption of the bone by the osteoclasts. This is especially relevant at sites where bone remodelling is most active.[7][8]The bisphosphonates have an intrinsic affinity for the calcium ions (hydroxyapatite) of the bone mineral just as the endogenous pyrophosphates. The difference lies in the non-hydrolysable carbon-phosphorus bond of the bisphosphonates which prevents their metabolism and at the same time ensure an effective absorption from the gastrointestinal tract.[9]
The primary inhibiting action of the first generation of bisphosphonates on osteoclasts is by inducing apoptosis. The mechanism of action is apparently by the formation of an ATP analogue or metabolite of the bisphosphonates like etidronic acid and clodronic acid. The ATP analogue accumulates in the cytosolof the osteoclast with a cytotoxic effect.[10]
The primary mechanism of action of the more developed nitrogen containing bisphosphonates is however by cellular effects on osteoclasts through inhibition of the mevalonate pathway and in particular the subsequent formation of isoprenoid lipids. The inhibition takes place at a key branch point in the pathway catalyzed by farnesyl pyrophosphate synthase (FPPS).[11] Isoprenoid lipids are necessary for post-translational modifications of small GTP-binding regulatory proteins like Rac, Rho and Ras of the Ras superfamily. The function of osteoclasts depends on them for a variety of cellular processes like apoptosis.[12]
Structure activity relationship[edit]
Pharmacophore[edit]
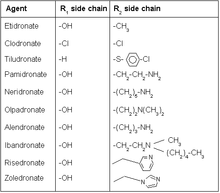
Bisphosphonates mimic the endogenous inorganic pyrophosphate where the oxygenbackbone is replaced with carbon (P-C-P for P-O-P). The two additional groups or side chains on the carbon backbone are usually referred to as R1 and R2. R1 is usually a hydroxyl group which enhances the affinity for the calcium by forming a tridentate ligandalong with the phosphate groups. The compound can be made more potent by optimizing the structure of the R2 group to best inhibit bone resorption.[13]
Phosphonate[edit]
Phosphonate groups in the chemical structure are important for the binding of the drug to the target enzyme. Studies have showed that removal or replacement of the phosphonate group with a carboxylic acid causes drastic loss in potency of the drug and the enzyme inhibitor no longer goes into an isomerized state.[14]
Hydroxyl group (R1 side chain)[edit]
Modification of the R1 side chain on bisphosphonates is very minor today, single hydroxyl group at that position seems to give the best results in terms of activity. The hydroxyl group plays a role in forming a water-induced bond with glutamine (Gln240) on the target enzyme. Drugs that have no hydroxyl group initially cause better inhibition than parent compounds, without hydroxyl group the drug seems to fit more easily into the open active site. The absence of hydroxyl group however reduces the ability to hold the target enzyme complex in isomerized state. Biological activity of bisphosphonates with hydroxyl group, therefore, appears over longer time.[14]
Nitrogen (R2 side chain)[edit]
Nitrogen containing bisphosphonates are the current most used drugs in the class because of their potency.[15] Studies have showed that nitrogen on bisphosphonates forms hydrogen bond with threonine (Thr201) and the carbonyl part of Lysine (Lys200) on target enzyme, therefore enhancing the binding of the complex. Altering the position of nitrogen can significantly change the ability for the nitrogen hydrogen bond to occur.[14]
| Bisphosphonate | potency (relative) |
|---|---|
| Alendronate | 1-5 |
| Risedronate | 10 |
| Zoledronate (IV) | 50 |
Modification of nitrogen containing side chain (R2 side chain)[edit]
Increased carbon length of the nitrogen R2 side chain alters activity. Side chain that is made out of three carbons has proven to be the most ideal length in terms of activity, increasing or decreasing the length of the chain from there has negative effect on biological activity. Alendronate, a common bisphosphonate drug, has a three carbon length side chain for example.[17] Risedronatehas heterocyclic structure containing nitrogen. Heterocyclic nitrogen containing bisphosphonates have revealed better results in terms of activity compared to earlier bisphosphonates with nitrogen bound to carbon chain. Studies on risedronate analogous with different placement of nitrogen on the ring have shown no measurable difference on biological activity. Increased length of carbon chain connected to the ring revealed negative results.[18] Zoledronate is the most potent bisphosphonate drug today only available as intravenous injection. It is the only bisphosphonate drug that has two nitrogen groups in the side chain hence its potency and route of administration differs from other drugs in the same class.[16]
References[edit]
- ^ a b Widler, Leo; Jaeggi, Knut A.; Glatt, Markus; Müller, Klaus; Bachmann, Rolf; Bisping, Michael; Born, Anne-Ruth; Cortesi, Reto; Guiglia, Gabriela; Jeker, Heidi; Klein, Rémy (2002-08-01). "Highly Potent Geminal Bisphosphonates. From Pamidronate Disodium (Aredia) to Zoledronic Acid (Zometa)". Journal of Medicinal Chemistry. 45 (17): 3721–3738. doi:10.1021/jm020819i. ISSN 0022-2623. PMID 12166945.
- ^ Reid, Ian R.; Brown, Jacques P.; Burckhardt, Peter; Horowitz, Zebulun; Richardson, Peter; Trechsel, Ulrich; Widmer, Albert; Devogelaer, Jean-Pierre; Kaufman, Jean-Marc; Jaeger, Philippe; Body, Jean-Jacques (2002-02-28). "Intravenous Zoledronic Acid in Postmenopausal Women with Low Bone Mineral Density". New England Journal of Medicine. 346 (9): 653–661. doi:10.1056/NEJMoa011807. ISSN 0028-4793.
- ^ a b Thompson, Keith; Rogers, Michael J. (2007-09-01). "The Molecular Mechanisms of Action of Bisphosphonates". Clinical Reviews in Bone and Mineral Metabolism. 5 (3): 130–144. doi:10.1007/s12018-007-9004-0. ISSN 1559-0119.
- ^ [1], "Bisphosphonate compounds and methods with enhanced potency for multiple targets including FPPS, GGPPS, and DPPS", issued 2008-04-11
- ^ Matthiesen, Robert A.; Varney, Michelle L.; Xu, Pauline C.; Rier, Alex S.; Wiemer, David F.; Holstein, Sarah A. (January 2018). "α-Methylation enhances the potency of isoprenoid triazole bisphosphonates as geranylgeranyl diphosphate synthase inhibitors". Bioorganic & Medicinal Chemistry. 26 (2): 376–385. doi:10.1016/j.bmc.2017.10.023. PMC 5752576. PMID 29248353.
- ^ Shimoda, Kazuma; Mitsuoka, Takahiro; Ueda, Kenta; Suemune, Hiroshi; Hirai, Go; Aso, Mariko (2018-12-19). "Synthesis of dendritic bisphosphonates as bone targeting ligands". Tetrahedron Letters. 59 (51): 4528–4531. doi:10.1016/j.tetlet.2018.11.028. ISSN 0040-4039.
- ^ Masarachia, P.; Weinreb, M.; Balena, R.; Rodan, G. A. (1996-09-01). "Comparison of the distribution of 3H-alendronate and 3H-Etidronate in rat and mouse bones". Bone. 19 (3): 281–290. doi:10.1016/8756-3282(96)00182-2. ISSN 8756-3282. PMID 8873969.
- ^ Sato, M; Grasser, W; Endo, N; Akins, R; Simmons, H; Thompson, D D; Golub, E; Rodan, G A (1991-12-01). "Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure". Journal of Clinical Investigation. 88 (6): 2095–2105. doi:10.1172/JCI115539. ISSN 0021-9738. PMC 295810. PMID 1661297.
- ^ Benford, Helena L.; Frith, Julie C.; Auriola, Seppo; Mönkkönen, Jukka; Rogers, Michael J. (1999-07-01). "Farnesol and Geranylgeraniol Prevent Activation of Caspases by Aminobisphosphonates: Biochemical Evidence for Two Distinct Pharmacological Classes of Bisphosphonate Drugs". Molecular Pharmacology. 56 (1): 131–140. doi:10.1124/mol.56.1.131. ISSN 0026-895X. PMID 10385693.
- ^ Selander, K. S.; Mönkkönen, J.; Karhukorpi, E. K.; Härkönen, P.; Hannuniemi, R.; Väänänen, H. K. (1996-11-01). "Characteristics of clodronate-induced apoptosis in osteoclasts and macrophages". Molecular Pharmacology. 50 (5): 1127–1138. ISSN 0026-895X. PMID 8913344.
- ^ Ebetino, Frank H.; Hogan, Anne-Marie L.; Sun, Shuting; Tsoumpra, Maria K.; Duan, Xuchen; Triffitt, James T.; Kwaasi, Aaron A.; Dunford, James E.; Barnett, Bobby L.; Oppermann, Udo; Lundy, Mark W. (2011-07-01). "The relationship between the chemistry and biological activity of the bisphosphonates". Bone. Bisphosphonates. 49 (1): 20–33. doi:10.1016/j.bone.2011.03.774. ISSN 8756-3282. PMID 21497677.
- ^ Coxon, F.P.; Rogers, M.J. (2003-01-01). "The Role of Prenylated Small GTP-Binding Proteins in the Regulation of Osteoclast Function". Calcified Tissue International. 72 (1): 80–84. doi:10.1007/s00223-002-2017-2. ISSN 0171-967X. PMID 12370802.
- ^ Widler, Leo; Jaeggi, Knut A.; Glatt, Markus; Müller, Klaus; Bachmann, Rolf; Bisping, Michael; Born, Anne-Ruth; Cortesi, Reto; Guiglia, Gabriela; Jeker, Heidi; Klein, Rémy (August 2002). "Highly Potent Geminal Bisphosphonates. From Pamidronate Disodium (Aredia) to Zoledronic Acid (Zometa)". Journal of Medicinal Chemistry. 45 (17): 3721–3738. doi:10.1021/jm020819i. ISSN 0022-2623.
- ^ a b c Dunford, James E.; Kwaasi, Aaron A.; Rogers, Michael J.; Barnett, Bobby L.; Ebetino, Frank H.; Russell, R. Graham G.; Oppermann, Udo; Kavanagh, Kathryn L. (April 2008). "Structure–Activity Relationships Among the Nitrogen Containing Bisphosphonates in Clinical Use and Other Analogues: Time-Dependent Inhibition of Human Farnesyl Pyrophosphate Synthase". Journal of Medicinal Chemistry. 51 (7): 2187–2195. doi:10.1021/jm7015733. ISSN 0022-2623. PMID 18327899.
- ^ Dunford, James E.; Kwaasi, Aaron A.; Rogers, Michael J.; Barnett, Bobby L.; Ebetino, Frank H.; Russell, R. Graham G.; Oppermann, Udo; Kavanagh, Kathryn L. (2008-04-01). "Structure–Activity Relationships Among the Nitrogen Containing Bisphosphonates in Clinical Use and Other Analogues: Time-Dependent Inhibition of Human Farnesyl Pyrophosphate Synthase". Journal of Medicinal Chemistry. 51 (7): 2187–2195. doi:10.1021/jm7015733. ISSN 0022-2623. PMID 18327899.
- ^ a b Tripathi, KD (2013), "Chapter-09 Adrenergic System and Drugs", Essentials of Medical Pharmacology, Jaypee Brothers Medical Publishers (P) Ltd., pp. 124–139, doi:10.5005/jp/books/12021_10, ISBN 9789350259375
- ^ Fleisch, Herbert (2002). "Development of bisphosphonates". Breast Cancer Research. 4 (1): 30–34. doi:10.1186/bcr414. ISSN 1465-5411. PMC 138713. PMID 11879557.
- ^ van Beek, E. R.; Löwik, C. W. G. M.; Ebetino, F. H.; Papapoulos, S. E. (1998-11-01). "Binding and antiresorptive properties of heterocycle-containing bisphosphonate analogs: structure-activity relationships". Bone. 23 (5): 437–442. doi:10.1016/S8756-3282(98)00120-3. ISSN 8756-3282. PMID 9823450.




No comments:
Post a Comment