Bone morphogenetic proteins (BMPs) are a group of growth factors also known as cytokines and as metabologens.[1] Originally discovered by their ability to induce the formation of bone and cartilage, BMPs are now considered to constitute a group of pivotal morphogenetic signals, orchestrating tissue architecture throughout the body.[2] The important functioning of BMP signals in physiology is emphasized by the multitude of roles for dysregulated BMP signalling in pathological processes. Cancerous disease often involves misregulation of the BMP signalling system. Absence of BMP signalling is, for instance, an important factor in the progression of colon cancer,[3] and conversely, overactivation of BMP signalling following reflux-induced esophagitis provokes Barrett's esophagus and is thus instrumental in the development of adenocarcinoma in the proximal portion of the gastrointestinal tract.[4]
Recombinant human BMPs (rhBMPs) are used in orthopedic applications such as spinal fusions, nonunions and oral surgery. rhBMP-2 and rhBMP-7 are Food and Drug Administration (FDA)-approved for some uses. rhBMP-2 causes more overgrown bone than any other BMPs and is widely used off-label.
Although rhBMP-2 and rhBMP-7 are used in the treatment of a variety of bone-related conditions including spinal fusions and nonunions, the risks of this off-label treatment are not understood.[9] While rhBMPs are approved for specific applications (spinal lumbar fusions with an anterior approach and tibia nonunions), up to 85% of all BMP usage is off-label.[9] rhBMP-2 is used extensively in other lumbar spinal fusion techniques (e.g., using a posterior approach, anterior or posterior cervical fusions[9]).
In 2001, the Food and Drug Administration (FDA) approved rhBMP-7 (a.k.a. OP-1; Stryker Biotech) for a humanitarian device exemption as an alternative to autograft in long bone nonunions.[9] In 2004, the humanitarian device exemption was extended as an alternative to autograft for posterolateral fusion.[9] In 2002, rhBMP-2 (Infuse; Medtronic) was approved for anterior lumbarinterbody fusions (ALIFs) with a lumbar fusion device.[9] In 2008 it was approved to repair posterolateral lumbar pseudarthrosis, open tibia shaft fractures with intramedullary nail fixation.[9] In these products, BMPs are delivered to the site of the fracture by being incorporated into a bone implant, and released gradually to allow bone formation, as the growth stimulation by BMPs must be localized and sustained for some weeks. The BMPs are eluted through a purified collagen matrix which is implanted in the site of the fracture.[5] rhBMP-2 helps grow bone better than any other rhBMP so it is much more widely used clinically.[5] There is "little debate or controversy" about the effectiveness of rhBMP-2 to grow bone to achieve spinal fusions,[5] and Medtronic generates $700 million in annual sales from their product.[10]
Contraindications[edit]
Bone morphogenetic protein (rhBMP) should not be routinely used in any type of anterior cervical spine fusion, such as with anterior cervical discectomy and fusion.[11] There are reports of this therapy causing swelling of soft tissue which in turn can cause life-threatening complications due to difficulty swallowing and pressure on the respiratory tract.[11]
BMPs interact with specific receptors on the cell surface, referred to as bone morphogenetic protein receptors (BMPRs).
Signal transduction through BMPRs results in mobilization of members of the SMAD family of proteins. The signaling pathways involving BMPs, BMPRs and SMADs are important in the development of the heart, central nervous system, and cartilage, as well as post-natal bone development.
They have an important role during embryonic development on the embryonic patterning and early skeletal formation. As such, disruption of BMP signaling can affect the body plan of the developing embryo. For example, BMP4 and its inhibitors noggin and chordin help regulate polarity of the embryo (i.e. back to front patterning). Specifically BMP-4 and its inhibitors play a major role in neurulation and the development of the neural plate. BMP-4 signals ectoderm cells to develop into skin cells, but the secretion of inhibitors by the underlying mesoderm blocks the action of BMP-4 to allow the ectoderm to continue on its normal course of neural cell development. Additionally, secretion of BMPs by the roof plate in the developing spinal cord helps to specify dorsal sensory interneurons.[12]
As a member of the transforming growth factor-beta superfamily, BMP signaling regulates a variety of embryonic patterning during fetal and embryonic development. For example, BMP signaling controls the early formation of the Mullerian duct (MD) which is a tubular structure in early embryonic developmental stage and eventually becomes female reproductive tracts. Chemical inhibiting BMP signals in chicken embryo caused a disruption of MD invagination and blocked the epithelial thickening of the MD-forming region, indicating that the BMP signals play a role in early MD development.[13] Moreover, BMP signaling is involved in the formation of foregut and hindgut,[14] intestinal villus patterning, and endocardial differentiation. Villi contribute to increase the effective absorption of nutrients by extending the surface area in small intestine. Gain or lose function of BMP signaling altered the patterning of clusters and emergence of villi in mouse intestinal model.[15] BMP signal derived from myocardium is also involved in endocardial differentiation during heart development. Inhibited BMP signal in zebrafish embryonic model caused strong reduction of endocardial differentiation, but only had little effect in myocardial development.[16] In addition, Notch-Wnt-Bmp crosstalk is required for radial patterning during mouse cochlea development via antagonizing manner.[17]
Mutations in BMPs and their inhibitors are associated with a number of human disorders which affect the skeleton.
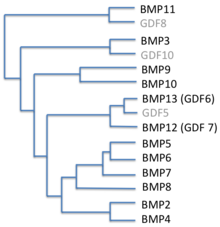
Several BMPs are also named 'cartilage-derived morphogenetic proteins' (CDMPs), while others are referred to as 'growth differentiation factors' (GDFs).
BMPs are also involved in adipogenesis and functional regulation of adipose tissue.[18] BMP4 favors white adipogenesis, whereas BMP7 activates brown fat functionality; BMP inhibitors are also involved in this regulation [18]
Types[edit]
Originally, seven such proteins were discovered. Of these, six (BMP2 through BMP7) belong to the Transforming growth factor beta superfamily of proteins. BMP1 is a metalloprotease. Since then, thirteen more BMPs, all of which are in the TGF-beta family, have been discovered, bringing the total to twenty.[5] The current nomenclature only recognizes 13, as many others are put under the growth differentiation factor naming instead.
| BMP | Known functions | Gene Locus |
|---|---|---|
| BMP1 | *BMP1 does not belong to the TGF-β family of proteins. It is a metalloprotease that acts on procollagen I, II, and III. It is involved in cartilage development. | Chromosome: 8; Location: 8p21 |
| BMP2 | Acts as a disulfide-linked homodimer and induces bone and cartilage formation. It is a candidate as a retinoid mediator. Plays a key role in osteoblast differentiation. | Chromosome: 20; Location: 20p12 |
| BMP3 | Induces bone formation. | Chromosome: 14; Location: 14p22 |
| BMP4 | Regulates the formation of teeth, limbs and bone from mesoderm. It also plays a role in fracture repair, epidermis formation, dorsal-ventral axis formation, and ovarian follical development. | Chromosome: 14; Location: 14q22-q23 |
| BMP5 | Performs functions in cartilage development. | Chromosome: 6; Location: 6p12.1 |
| BMP6 | Plays a role in joint integrity in adults. Controls iron homeostasis via regulation of hepcidin. | Chromosome: 6; Location: 6p12.1 |
| BMP7 | Plays a key role in osteoblast differentiation. It also induces the production of SMAD1. Also key in renal development and repair. | Chromosome: 20; Location: 20q13 |
| BMP8a | Involved in bone and cartilage development. | Chromosome: 1; Location: 1p35–p32 |
| BMP8b | Expressed in the hippocampus. | Chromosome: 1; Location: 1p35–p32 |
| BMP10 | May play a role in the trabeculation of the embryonic heart. | Chromosome: 2; Location: 2p14 |
| BMP11 | Controls anterior-posterior patterning. | Chromosome: 12; Location: 12p |
| BMP15 | May play a role in oocyte and follicular development. | Chromosome: X; Location: Xp11.2 |
History[edit]
From the time of Hippocrates it has been known that bone has considerable potential for regeneration and repair. Nicholas Senn, a surgeon at Rush Medical College in Chicago, described the utility of antiseptic decalcified bone implants in the treatment of osteomyelitisand certain bone deformities.[20] Pierre Lacroix proposed that there might be a hypothetical substance, osteogenin, that might initiate bone growth.[21]
The biological basis of bone morphogenesis was shown by Marshall R. Urist. Urist made the key discovery that demineralized, lyophilized segments of bone induced new bone formation when implanted in muscle pouches in rabbits. This discovery was published in 1965 by Urist in Science.[22] Urist proposed the name "Bone Morphogenetic Protein" in the scientific literature in the Journal of Dental Research in 1971.[23]
Bone induction is a sequential multistep cascade. The key steps in this cascade are chemotaxis, mitosis, and differentiation. Early studies by Hari Reddi unraveled the sequence of events involved in bone matrix-induced bone morphogenesis.[24] On the basis of the above work, it seemed likely that morphogens were present in the bone matrix. Using a battery of bioassays for bone formation, a systematic study was undertaken to isolate and purify putative bone morphogenetic proteins.
A major stumbling block to purification was the insolubility of demineralized bone matrix. To overcome this hurdle, Hari Reddi and Kuber Sampath used dissociative extractants, such as 4M guanidine HCL, 8M urea, or 1% SDS.[25] The soluble extract alone or the insoluble residues alone were incapable of new bone induction. This work suggested that the optimal osteogenic activity requires a synergy between soluble extract and the insoluble collagenous substratum. It not only represented a significant advance toward the final purification of bone morphogenetic proteins by the Reddi laboratory,[26][27] but ultimately also enabled the cloning of BMPs by John Wozney and colleagues at Genetics Institute.[28]
https://en.wikipedia.org/wiki/Bone_morphogenetic_protein
Further reading[edit]
- Reddi AH (1997). "Bone morphogenetic proteins: an unconventional approach to isolation of first mammalian morphogens". Cytokine Growth Factor Rev. 8 (1): 11–20. doi:10.1016/S1359-6101(96)00049-4. PMID 9174660.
- Bessa PC, Casal M, Reis RL (Jan 2008). "Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts)". Journal of Tissue Engineering and Regenerative Medicine. 2 (1): 1–13. doi:10.1002/term.63. hdl:1822/13420. PMID 18293427. S2CID 13038950. Archived from the original on 2012-10-18.
External links[edit]
| Wikimedia Commons has media related to Bone morphogenetic proteins. |
- BMP: The What and the Who
- BMPedia - the Bone Morphogenetic Protein Wiki
- Bone+Morphogenetic+Proteins at the US National Library of Medicine Medical Subject Headings (MeSH)
- Chen D, Zhao M, Mundy GR (Dec 2004). "Bone morphogenetic proteins". Growth Factors (Chur, Switzerland). 22 (4): 233–241. doi:10.1080/08977190412331279890. PMID 15621726. S2CID 22932278.
- Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatkowski JP, Park JY, He TC (Aug 2003). "Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs)". The Journal of Bone and Joint Surgery. American Volume. 85-A (8): 1544–52. doi:10.2106/00004623-200308000-00017. PMID 12925636.link
Cell signaling: TGFβ signaling pathway
showvte
Drugs for treatment of bone diseases (M05)
showvte
TGFβ receptor superfamily modulators
Categories: Bone morphogenetic proteinGrowth factorsAnimal developmental biologyProteins
https://en.wikipedia.org/wiki/Bone_morphogenetic_protein
https://en.wikipedia.org/wiki/Monoamine_reuptake_inhibitor
https://en.wikipedia.org/wiki/Amineptine
https://en.wikipedia.org/wiki/Neurotoxin
https://en.wikipedia.org/wiki/Category:Hormonal_antineoplastic_drugs
https://en.wikipedia.org/wiki/Cytolysis
https://en.wikipedia.org/wiki/Category:Dibenzocycloheptenes
https://en.wikipedia.org/wiki/Astrocyte
https://en.wikipedia.org/wiki/Stimulant#Methamphetamine
https://en.wikipedia.org/wiki/TGF_beta_signaling_pathway
https://en.wikipedia.org/wiki/Bone_disease
https://en.wikipedia.org/wiki/ATC_code_M05
https://en.wikipedia.org/wiki/TGF-beta_receptor_family
https://en.wikipedia.org/wiki/Receptor_modulator
https://en.wikipedia.org/wiki/Chordin
https://en.wikipedia.org/wiki/Cripto
https://en.wikipedia.org/wiki/Noggin_(protein)
https://en.wikipedia.org/wiki/Follistatin
https://en.wikipedia.org/wiki/Cerberus_(protein)
https://en.wikipedia.org/wiki/Lefty_(protein)
https://en.wikipedia.org/wiki/Mothers_against_decapentaplegic_homolog_6
https://en.wikipedia.org/wiki/Mothers_against_decapentaplegic_homolog_9
https://en.wikipedia.org/wiki/R-SMAD
https://en.wikipedia.org/wiki/Anti-Müllerian_hormone
https://en.wikipedia.org/wiki/Activin_type_1_receptors
https://en.wikipedia.org/wiki/Bone_morphogenetic_protein_10
https://en.wikipedia.org/wiki/Bone_morphogenetic_protein_6
https://en.wikipedia.org/wiki/Bone_morphogenetic_protein_9
https://en.wikipedia.org/wiki/Activin_and_inhibin
https://en.wikipedia.org/wiki/Growth_differentiation_factor-9
https://en.wikipedia.org/wiki/GDF10
https://en.wikipedia.org/wiki/GDF6
016910
The transforming growth factor beta (TGF-β) family is a large group of structurally related cell regulatory proteins that was named after its first member, TGF-β1, originally described in 1983.[2] They interact with TGF-beta receptors.
Many proteins have since been described as members of the TGF-β family in a variety of species, including invertebrates as well as vertebrates and categorized into 23 distinct gene types that fall into four major subfamilies:[3][4][5]
- The TGF-β subfamily
- The bone morphogenetic proteins and the growth differentiation factors
- The activin and inhibin subfamilies
- The Left-right determination factors
- A group encompassing various divergent members
Transforming growth factor-beta (TGF-beta)[6] is a multifunctional peptide that controls proliferation, differentiation and other functions in many cell types. TGF-beta-1 is a peptide of 112 amino acid residues derived by proteolytic cleavage from the C-terminal of a precursor protein. These proteins interact with a conserved family of cell surface serine/threonine-specific protein kinase receptors, and generate intracellular signals using a conserved family of proteins called SMADs. They play fundamental roles in the regulation of basic biological processes such as growth, development, tissue homeostasis and regulation of the immune system.[3]
https://en.wikipedia.org/wiki/Transforming_growth_factor_beta_family
https://en.wikipedia.org/wiki/Category:Animal_developmental_biology
Endochondral ossification[1][2] is one of the two essential processes during fetal development of the mammalian skeletal system by which bone tissue is created. Unlike intramembranous ossification, which is the other process by which bone tissue is created, cartilage is present during endochondral ossification. Endochondral ossification is also an essential process during the rudimentary formation of long bones,[3] the growth of the length of long bones,[4] and the natural healing of bone fractures.[5]
https://en.wikipedia.org/wiki/Endochondral_ossification
1q21.1 copy number variations (CNVs)[1]are rare aberrations of human chromosome 1.
In a common situation a human cell has one pair of identical chromosomes on chromosome 1. With the 1q21.1 CNVs one chromosome of the pair is not complete because a part of the sequence of the chromosome is missing, or overcomplete, because some parts of the sequence are duplicated. The result is that one chromosome is of normal length and the other one is too long or too short.
https://en.wikipedia.org/wiki/1q21.1_copy_number_variations
1q21.1 deletion syndrome is a rare aberration of chromosome 1. A human cell has one pair of identical chromosomes on chromosome 1. With the 1q21.1 deletion syndrome, one chromosome of the pair is not complete, because a part of the sequence of the chromosome is missing. One chromosome has the normal length and the other is too short.
In 1q21.1, the '1' stands for chromosome 1, the 'q' stands for the long arm of the chromosome and '21.1' stands for the part of the long arm in which the deletion is situated.
The syndrome is a form of the 1q21.1 copy number variations, and it is a deletion in the distal area of the 1q21.1 part. The CNV leads to a very variable phenotype, and the manifestations in individuals are quite variable. Some people who have the syndrome can function in a normal way, while others have symptoms of mental retardation and various physical anomalies.
1q21.1 microdeletion is a very rare chromosomal condition. Only 46 individuals with this deletion have been reported in medical literature as of August 2011.[1]
https://en.wikipedia.org/wiki/1q21.1_deletion_syndrome
Ateliosis or ateleiosis is a diagnosis used in the early 1900s to describe patients with short stature. Ateliosis literally means "failure to achieve perfection", and was used to describe proportional dwarfism.[1] The term was popularised by Hastings Gilford, who used the term to refer to forms of dwarfism associated with and without sexual maturation.[2]
Ateliosis was reported as early as 1904 in relation to progeria, a syndrome of premature aging.[3]
According to the Merriam-Webster Dictionary, it is “dwarfism associated with anterior pituitary deficiencies and marked by essentially normal intelligence and proportions though often retarded sexual development”.[4] The physical characteristics include: normal facial features, childlike high pitched voice, proportioned body, and abnormal genitalia. Their mental development is normal to slightly delayed. Hastings Gilford originated the term to describe patients with "continuous youth".[5]
https://en.wikipedia.org/wiki/Ateliosis
https://en.wikipedia.org/wiki/Giant_depolarizing_potential
https://en.wikipedia.org/wiki/Subgranular_zone
https://en.wikipedia.org/wiki/Subplate
https://en.wikipedia.org/wiki/Guidepost_cells
https://en.wikipedia.org/wiki/Growth_cone
https://en.wikipedia.org/wiki/Germinal_matrix
https://en.wikipedia.org/wiki/Bone_morphogenetic_protein
https://en.wikipedia.org/wiki/Monoamine_reuptake_inhibitor
https://en.wikipedia.org/wiki/Phosphorus_trioxide
https://en.wikipedia.org/wiki/Astrocyte
https://en.wikipedia.org/wiki/Stimulant#Methamphetamine
https://en.wikipedia.org/wiki/Phosphorous_acid
https://en.wikipedia.org/wiki/Cytolysis
https://en.wikipedia.org/wiki/Phosphorous
https://en.wikipedia.org/wiki/Role_of_cell_adhesions_in_neural_development
https://en.wikipedia.org/wiki/Rostral_migratory_stream
connexins junctions matricing matrix components (usu prot, particle, phosphorous; scale/level/etc.)
https://en.wikipedia.org/wiki/Slit_(protein)
https://en.wikipedia.org/wiki/SIX3
https://en.wikipedia.org/wiki/Radial_glial_cell
https://en.wikipedia.org/wiki/Sensory_maps_and_brain_development
https://en.wikipedia.org/wiki/Segmentation_in_the_human_nervous_system
https://en.wikipedia.org/wiki/Ganglion_mother_cell
https://en.wikipedia.org/wiki/Subependymal_zone
https://en.wikipedia.org/wiki/Subventricular_zone
https://en.wikipedia.org/wiki/Synaptogenesis
https://en.wikipedia.org/wiki/Gyrification
https://en.wikipedia.org/wiki/Floor_plate
https://en.wikipedia.org/wiki/Follower_neuron
https://en.wikipedia.org/wiki/Category:Developmental_neuroscience
https://en.wikipedia.org/wiki/RASopathy
Misters
https://en.wikipedia.org/wiki/Dendritic_filopodia
macrophage leukocyte amoeba immune system principles of embryology life macrophage phagocyte leukocyte white transparent clear etc.
https://en.wikipedia.org/wiki/Developmental_regression
https://en.wikipedia.org/wiki/Pioneer_axon
https://en.wikipedia.org/wiki/Pioneer_neuron
https://en.wikipedia.org/wiki/NKX_2-9
https://en.wikipedia.org/wiki/Nodal_signaling_pathway
https://en.wikipedia.org/wiki/Neuropoiesis
https://en.wikipedia.org/wiki/Neurosphere
Nkx 2.9 is a transcription factor responsible for the formation of the branchial and visceral motor neuron subtypes of cranial motor nerves in vertebrates. Nkx 2.9 works together with another transcription factor, Nkx 2.2, to direct neural progenitor cells to their cell fate.[1]
https://en.wikipedia.org/wiki/NKX_2-9
https://en.wikipedia.org/wiki/Cerebral_organoid
https://en.wikipedia.org/wiki/Development_of_the_nervous_system
https://en.wikipedia.org/wiki/Axon_guidance
https://en.wikipedia.org/wiki/Arrested_development
https://en.wikipedia.org/wiki/Netrin
https://en.wikipedia.org/wiki/MuSK_protein
https://en.wikipedia.org/wiki/Myelinogenesis
https://en.wikipedia.org/wiki/Category:Developmental_neuroscience
The neuregulin family includes:
- Neuregulin-1 (NRG1), with numerous discovered isoforms stemming from alternative splicing:
- Type I NRG1; alternative names: Heregulin, NEU differentiation factor (NDF), or acetylcholine receptor inducing activity (ARIA)
- Type II NRG1; alternative name: Glial Growth Factor-2 (GGF2);
- Type III NRG1; alternative name: Sensory and motor neuron-derived factor (SMDF);
- Type IV NRG1;
- Type V NRG1;
- Type VI NRG1; Types IV-VI are proteins with 3 novel N-terminal domains identified in 2004.[5]
- Neuregulin-2 (NRG2);
- Neuregulin-3 (NRG3);
- Neuregulin-4 (NRG4);
In mammals, neuregulin family members are the products of 4 genes NRG1, NRG2, NRG3 and NRG4 respectively.
The transmembrane forms of neuregulin 1 (NRG1) are present within synaptic vesicles, including those containing glutamate.[6]After exocytosis, NRG1 is in the presynaptic membrane, where the ectodomain of NRG1 may be cleaved off. The ectodomain then migrates across the synaptic cleft and binds to and activates a member of the EGF-receptor family on the postsynaptic membrane. This has been shown to increase the expression of certain glutamate-receptor subunits. NRG1 appears to signal for glutamate-receptor subunit expression, localization, and /or phosphorylation facilitating subsequent glutamate transmission.
The NRG1 gene has been identified as a potential gene determining susceptibility to schizophrenia by a combination of genetic linkage and association approaches.[6]
NRG1[edit]
NRG1 plays a role in synapse development, influencing the upregulation of acetylcholine receptor genes beneath the endplate after mammalian motor neurons have made synaptic contact with muscle fibres, hence its alternative name ARIA = Acetylcholine Receptor Inducing Activity.
Animal models[edit]
A study done on mice in early 2009 has indicated that when neuregulin-1\ErbB signalling is disrupted, the dendritic spines of neurons grow but do not fully form. This produced no immediate noticeable changes to brain development, but in adults there was a reduction of dendritic spines on neurons.[7][8] Glutamatergic signalling was markedly disrupted in the mice as a result of the experiment.
In fish, birds, and earthworms[edit]
NRG-1,2,3 have been found in fish and birds.
mRNA similar to mammalian Pro-NRG2 precursor has been found in humus earthworm Lumbricidae.
https://en.wikipedia.org/wiki/Neuregulin
Disease Increase Glu / neuroregulins
The following steps are followed in the conversion of cartilage to bone:
- Zone of reserve cartilage. This region, farthest from the marrow cavity, consists of typical hyaline cartilage that as yet shows no sign of transforming into bone.[37]
- Zone of cell proliferation. A little closer to the marrow cavity, chondrocytes multiply and arrange themselves into longitudinal columns of flattened lacunae.[37]
- Zone of cell hypertrophy. Next, the chondrocytes cease to divide and begin to hypertrophy (enlarge), much like they do in the primary ossification center of the fetus. The walls of the matrix between lacunae become very thin.[37]
- Zone of calcification. Minerals are deposited in the matrix between the columns of lacunae and calcify the cartilage. These are not the permanent mineral deposits of bone, but only a temporary support for the cartilage that would otherwise soon be weakened by the breakdown of the enlarged lacunae.[37]
- Zone of bone deposition. Within each column, the walls between the lacunae break down and the chondrocytes die. This converts each column into a longitudinal channel, which is immediately invaded by blood vessels and marrow from the marrow cavity. Osteoblasts line up along the walls of these channels and begin depositing concentric lamellae of matrix, while osteoclasts dissolve the temporarily calcified cartilage.[37]
The cancellous part of bones contain bone marrow. Bone marrow produces blood cells in a process called hematopoiesis.[40]Blood cells that are created in bone marrow include red blood cells, platelets and white blood cells.[41] Progenitor cells such as the hematopoietic stem cell divide in a process called mitosis to produce precursor cells. These include precursors which eventually give rise to white blood cells, and erythroblasts which give rise to red blood cells.[42] Unlike red and white blood cells, created by mitosis, platelets are shed from very large cells called megakaryocytes.[43] This process of progressive differentiation occurs within the bone marrow. After the cells are matured, they enter the circulation.[44] Every day, over 2.5 billion red blood cells and platelets, and 50–100 billion granulocytes are produced in this way.[15]
As well as creating cells, bone marrow is also one of the major sites where defective or aged red blood cells are destroyed.[15]
Metabolic[edit]
- Mineral storage – bones act as reserves of minerals important for the body, most notably calcium and phosphorus.[45][citation needed][46]
Determined by the species, age, and the type of bone, bone cells make up to 15 percent of the bone. Growth factor storage—mineralized bone matrix stores important growth factors such as insulin-like growth factors, transforming growth factor, bone morphogenetic proteins and others.[47]
- Fat storage – marrow adipose tissue (MAT) acts as a storage reserve of fatty acids.[48]
- Acid-base balance – bone buffers the blood against excessive pH changes by absorbing or releasing alkaline salts.[49]
- Detoxification – bone tissues can also store heavy metals and other foreign elements, removing them from the blood and reducing their effects on other tissues. These can later be gradually released for excretion.[50]
- Endocrine organ – bone controls phosphate metabolism by releasing fibroblast growth factor 23 (FGF-23), which acts on kidneys to reduce phosphate reabsorption. Bone cells also release a hormone called osteocalcin, which contributes to the regulation of blood sugar (glucose) and fat deposition. Osteocalcin increases both the insulin secretion and sensitivity, in addition to boosting the number of insulin-producing cells and reducing stores of fat.[51]
- Calcium balance – the process of bone resorption by the osteoclasts releases stored calcium into the systemic circulation and is an important process in regulating calcium balance. As bone formation actively fixes circulating calcium in its mineral form, removing it from the bloodstream, resorption actively unfixes it thereby increasing circulating calcium levels. These processes occur in tandem at site-specific locations.[52]
Tumours[edit]
There are several types of tumour that can affect bone; examples of benign bone tumours include osteoma, osteoid osteoma, osteochondroma, osteoblastoma, enchondroma, giant cell tumour of bone, and aneurysmal bone cyst.[64]
Cancer[edit]
Cancer can arise in bone tissue, and bones are also a common site for other cancers to spread (metastasise) to.[65] Cancers that arise in bone are called "primary" cancers, although such cancers are rare.[65] Metastases within bone are "secondary" cancers, with the most common being breast cancer, lung cancer, prostate cancer, thyroid cancer, and kidney cancer.[65] Secondary cancers that affect bone can either destroy bone (called a "lytic" cancer) or create bone (a "sclerotic" cancer). Cancers of the bone marrow inside the bone can also affect bone tissue, examples including leukemia and multiple myeloma. Bone may also be affected by cancers in other parts of the body. Cancers in other parts of the body may release parathyroid hormone or parathyroid hormone-related peptide. This increases bone reabsorption, and can lead to bone fractures.
Bone tissue that is destroyed or altered as a result of cancers is distorted, weakened, and more prone to fracture. This may lead to compression of the spinal cord, destruction of the marrow resulting in bruising, bleeding and immunosuppression, and is one cause of bone pain. If the cancer is metastatic, then there might be other symptoms depending on the site of the original cancer. Some bone cancers can also be felt.
Cancers of the bone are managed according to their type, their stage, prognosis, and what symptoms they cause. Many primary cancers of bone are treated with radiotherapy. Cancers of bone marrow may be treated with chemotherapy, and other forms of targeted therapy such as immunotherapy may be used.[66] Palliative care, which focuses on maximising a person's quality of life, may play a role in management, particularly if the likelihood of survival within five years is poor.
Other painful conditions[edit]
- Osteomyelitis is inflammation of the bone or bone marrow due to bacterial infection.[67]
- Osteomalacia is a painful softening of adult bone caused by severe vitamin D deficiency.[68]
- Osteogenesis imperfecta[69]
- Osteochondritis dissecans[70]
- Ankylosing spondylitis[71]
- Skeletal fluorosis is a bone disease caused by an excessive accumulation of fluoride in the bones. In advanced cases, skeletal fluorosis damages bones and joints and is painful.[72]
https://en.wikipedia.org/wiki/Bone#Development
https://en.wikipedia.org/wiki/Biomechanics
Distraction osteogenesis (DO), also called callus distraction, callotasis and osteodistraction, is a process used in orthopedic surgery, podiatric surgery, and oral and maxillofacial surgery to repair skeletal deformities and in reconstructive surgery.[1][2] The procedure involves cutting and slowly separating bone, allowing the bone healing process to fill in the gap.[3]
https://en.wikipedia.org/wiki/Distraction_osteogenesis
Artificial bone refers to bone-like material created in a laboratory that can be used in bone grafts, to replace human bone that was lost due to severe fractures, disease, etc.[1]
Bone fracture, which is a complete or partial break in the bone, is a very common condition that has more than three million US cases per year.[2] Human bones have the ability to regenerate themselves by cycle of bone resorption and bone formation. The cell responsible for bone resorption is osteoclast, while the cell responsible for bone formation is osteoblast. That being said, the human body can regenerate fractured bone. However, if damage to bone is caused by a disease or severe injury, it becomes difficult for the body to repair itself. When the human body is unable to regenerate the lost bone tissue, surgeons come in and replace the missing bone using autografts, allografts, and synthetic grafts (artificial bone). When comparing artificial bone to autograft and allograft, it is less invasive and more biocompatible since it avoids the risk of unknown viral infections.[3]
When designing implanted biomaterials, key criteria are biocompatibility, osteoconductivity, high porosityand biomechanics compatibility. Artificial bone was initially made of materials like metals and solid ceramics, which are strong enough to sustain the loading in bone. However, the rigidity of those materials created an enormous burden on patients and was not consistent with the criteria for implanting biomaterials. Artificial bones made of metal and ceramic tend to do poorly in terms of biocompatibility since it is difficult to blend into bone tissues.[4] Thus, to better help those in need to live a more comfortable life, engineers have been developing new techniques to produce and design better artificial bone structure and material.
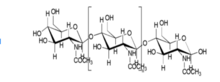
The two major components of bone are hydroxyapatite [Ca10(PO4)6(OH)2], and collagen fibers. Hydroxyapatite, which is one of the most stable forms of calcium phosphate, makes up about 60 to 65 percent of the bone.[5] The rest of the bone is composed of materials including chondroitin sulfate, keratan sulfate and lipid.[5] Increased research and knowledge regarding the organization, structure of properties of collagen and hydroxyapatite have led to many developments in collagen-based scaffolds in bone tissue engineering. The structure of hydroxyapatite is very similar to that of the original bone, and collagen can act as molecular cables and further improve the biocompatibility of the implant.[6]
https://en.wikipedia.org/wiki/Artificial_bone
Current areas of bone grafting (types of bones, composites)[edit]
Research on material types in bone grafting has been traditionally centered on producing composites of organic polysaccharides (chitin, chitosan, alginate) and minerals (hydroxyapatite). Alginate scaffolds, composed of cross-linked calcium ions, are actively being explored in the regeneration of skin, liver, and bone.[9] Alginate's ability to scaffold and makes it a novel polysaccharide. Even though many minerals can be adapted for bone composition, hydroxyapatite remains the dominant material, as its strength and the known Jager-Fratzl model of human bone provide a pre-existing framework for spacing and fabrication.
Material types[edit]
Materials suited for use in artificial bones need to be biocompatible, osteoconductive, and mechanically strong.[5] Hydroxyapatite is often used in artificial bone studies because it has the biocompatibility and osteoconductivity required for an effective, long-lasting bone implant, but is quite brittle,[5] and further exhibits a dissolution rate of about 10 wt% per year, which is significantly slower than the growth rate of newly formed bone, necessitating measures to enhance its dissolution rate.[10] For applications that require a material with better toughness, nanostructured artificial nacre may be used due to its high tensile strength and Young's modulus.[11] In many cases, using one type of material limits the capabilities of an artificial bone implant, so composites are utilized. Implants composed of chitosan and hydroxyapatite take advantage of chitosan's biocompatibility and its ability to be molded into complex porous shapes as well as hydroxyapatite's osteoconductivity to create a composite that features all three traits.[5] Other composites suitable for use in artificial bone are those using alginate, a biopolymer known for its scaffold-forming properties. Uses for alginate in composites include chitosan composites for bone tissue repair, bioglass composites for repairing or replacing defective or diseased bone, or ceramic-collagen composites for bone regeneration.[9] The material used in an artificial bone implant ultimately depends on the type of implant being created and its use.
Inkjet printing of artificial bones
HA nanocrystals are synthesized by wet synthesis using diammonium phosphate and calcium chloride as phosphorus and calcium precursors, respectively.[12]
Effective bone substitute materials should exhibit good mechanical strength along with adequate bioactivity. Bioactivity, which is often gauged in terms of dissolution rates and the formation of a mineral layer on the implant surface in-vivo, can be enhanced in biomaterials, in particular hydroxyapatite, by modifying the composition and structure by doping.[10] As an alternative to hydroxyaptatite systems, Chitosan composites have been thoroughly studied as one material to use for artificial bone.[5] Chitosan by itself can be easily modified into complex shapes that include porous structures, making it suitable for cell growth and osteoconduction.[5] In addition, chitosan scaffolds are biocompatible and biodegradable, but have low toughness, and the material itself is not osteoconductive.[5] Hydroxyapatite, on the other hand, features excellent biocompatibility but is hindered by its brittle nature.[14] When implemented with hydroxyapatite as a composite, both the toughness and osteoconductivity significantly improve, making the composite a viable option for material for artificial bone.[5] Chitosan can also be used with carbon nanotubes, which have a high Young's modulus (1.0–1.8 TPa), tensile strength (30–200 GPa), elongation at break (10–30%), and aspect ratio (>1,000).[5] Carbon nanotubes are very small in size, chemically and structurally stable, and bioactive.[5] The composite formed by carbon nanotubes and chitosan greatly improves the toughness of chitosan.[5] Nanostructured artificial nacre is another option for creating artificial bone.[11] Natural nacre is composed of an arrangement of organic and inorganic layers similar to brick and mortar.[9] This along with the ionic crosslinking of tightly folded molecules allow nacre to have high strength and toughness.[9]Artificial nacre that mimicked both the structure and the effect of the ionic bonds had a tensile strength similar to natural nacre as well as an ultimate Young's modulus similar to lamellar bone.[11] From a mechanical standpoint, this material would be a viable option for artificial bone.
Research on artificial bone materials has revealed that bioactive and resorbable silicate glasses (bioglass), glass-ceramics, and calcium phosphates exhibit mechanical properties that are similar to human bone.[18] Similar mechanical properties do not assure biocompatibility. The body's biological response to those materials depends on many parameters including chemical composition, topography, porosity, and grain size.[18] If the material is metal, there is a risk of corrosion and infection. If the material is ceramic, it is difficult to form the desired shape, and bone can't reabsorb or replace it due to its high crystallinity.[3] Hydroxyapatite, on the other hand, has shown excellent properties in supporting the adhesion, differentiation, and proliferation of osteogenesis cell since it is both thermodynamically stable and bioactive.[18] Artificial bones using hydroxyapatite combine with collagen tissue helps to form new bones in pores, and have a strong affinity to biological tissues while maintaining uniformity with adjacent bone tissue.[3]Despite its excellent performance in interacting with bone tissue, hydroxyapatite has the same problem as ceramic in reabsorption due to its high crystallinity. Since hydroxyapatite is processed at a high temperature, it is unlikely that it will remain in a stable state.[3]
https://en.wikipedia.org/wiki/Artificial_bone



No comments:
Post a Comment