Photochemical smog[edit]
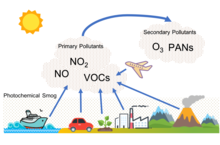
Photochemical smog, often referred to as "summer smog", is the chemical reaction of sunlight, nitrogen oxides and volatile organic compounds in the atmosphere, which leaves airborne particles and ground-level ozone.[19] Photochemical smog depends on primary pollutants as well as the formation of secondary pollutants. These primary pollutants include nitrogen oxides, particularly nitric oxide (NO) and nitrogen dioxide (NO2), and volatile organic compounds. The relevant secondary pollutants include peroxylacyl nitrates(PAN), tropospheric ozone, and aldehydes. An important secondary pollutant for photochemical smog is ozone, which is formed when hydrocarbons (HC) and nitrogen oxides (NOx) combine in the presence of sunlight; nitrogen dioxide (NO2), which is formed as nitric oxide (NO) combines with oxygen (O2) in the air.[20] In addition, when SO2 and NOx are emitted they eventually are oxidized in the troposphere to nitric acid and sulfuric acid, which, when mixed with water, form the main components of acid rain.[21] All of these harsh chemicals are usually highly reactive and oxidizing. Photochemical smog is therefore considered to be a problem of modern industrialization. It is present in all modern cities, but it is more common in cities with sunny, warm, dry climates and a large number of motor vehicles.[22] Because it travels with the wind, it can affect sparsely populated areas as well.
The composition and chemical reactions involved in photochemical smog were not understood until the 1950s. In 1948, flavor chemist Arie Haagen-Smit adapted some of his equipment to collect chemicals from polluted air, and identified ozone as a component of Los Angeles smog. Haagen-Smit went on to discover that nitrogen oxides from automotive exhausts and gaseous hydrocarbons from cars and oil refineries, exposed to sunlight, were key ingredients in the formation of ozone and photochemical smog.[23]:219–224[24][25]Haagen-Smit worked with Arnold Beckman, who developed various equipment for detecting smog, ranging from an "Apparatus for recording gas concentrations in the atmosphere" patented on 7 October 1952, to "air quality monitoring vans" for use by government and industry.[23]:224–226
https://en.wikipedia.org/wiki/Smog#Photochemical_smog

No comments:
Post a Comment