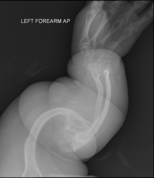
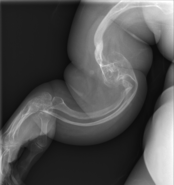
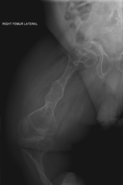
Osteogenesis imperfecta (IPA: /ˌɒstioʊˈdʒɛnəsɪs ˌɪmpɜːrˈfɛktə/;[4][5] OI), also known as brittle bone disease, is a group of genetic disorders that mainly affect the bones.[2][1]:85 It results in bones that break easily.[2] The range of symptoms may be mild to severe.[8]:1512 Symptoms found in various types of OI include a blue tint to the whites of the eye (sclerae), short stature, loose joints, hearing loss, breathing problems[6] and problems with the teeth (dentinogenesis imperfecta).[8]
Potentially life-threatening complications, all of which become more common in more severe OI, include: tearing (dissection) of the major arteries, such as the aorta;[1]:333[9] pulmonary insufficiency secondary to distortion of the ribcage;[1]:335–341[10] and basilar invagination.[11]:106–107
The underlying mechanism is usually a problem with connective tissue due to a lack of, or poorly formed, type I collagen.[8]:1513 In more than 90% of cases, OI occurs due to mutations in the COL1A1 or COL1A2 genes.[2] These genetic problems may be inherited from a person's parents in an autosomal dominant manner, but may also occur via a new mutation—de novo.[2][12]There are four main types, with type I being the least severe and type II the most severe.[2] As of August 2021, 19 different genes are known to cause the 21 documented types of OI.[13][14] Diagnosis is often based on symptoms and may be confirmed by a collagen biopsy and/or a DNA test.[6]
Although there is no cure,[6] OI does not have a major effect on life expectancy,[1]:461[12] and many people with OI can achieve a significant agree of autonomy.[15] Maintaining a healthy lifestyle by exercising and avoiding smoking can help prevent fractures.[6] Treatment may include acute care of broken bones, pain medication, physical therapy, mobility aids such as leg braces or wheelchairs, and rodding surgery,[6] a type of surgery that puts metal intramedullary rods along the long bones (such as the femur) in an attempt to strengthen them.[6] Evidence also supports the use of medications of the bisphosphonate class, such as pamidronate, to increase bone density.[16] Bisphosphonates are especially effective in children,[17] however it is unclear if they lead to increases in quality of life or decrease the incidence of fractures.[7]
OI affects about one in 10,000 to 20,000 people.[2] Outcomes depend on the genetic cause of the disorder (its type), but death during childhood is rare.[6]Moderate to severe OI primarily affects mobility; if rodding surgery is performed during childhood, some of those with more severe types of OI may gain the ability to walk.[18] The condition has been described since ancient history.[19] The Latinate term osteogenesis imperfecta came into use in 1849 and literally translates to "imperfect bone formation".[19][20]:683
Type I collagen is present all throughout the circulatory and respiratory systems: from the ventricles of the heart itself, to the heart valves, to the vasculature,[1]:329 and as an integral part of the connective tissue of the lungs.[1]:336 As such, cardiovascular complications, among them aortic insufficiency, aortic aneurysm, and arterial dissections, are sometimes comorbid with OI,[1]:333but not as often comorbid as with Marfan syndrome.[1]:332
Respiratory illnesses are a major cause of death in OI.[10][1]:335 The most obvious source of respiratory problems in OI is pulmonary insufficiency caused by problems in the architecture of the thoracic wall.[1]:341 However, respiratory tract infections, such as pneumonia, are also more fatal among those with OI than the general population.[10][32] Those with more severe ribcagedeformities were found to have worse lung restriction in a small-scale 2012 study involving 22 Italian patients with OI types III and IV, plus 26 non-affected controls.[10]
| Osteogenesis imperfecta (OI) | |
|---|---|
| Other names | Brittle bone disease,[1] Lobstein syndrome,[1]:5 fragilitas ossium,[2] Vrolik disease,[1]:5 osteopsathyrosis idiopathica[3]:347 |
 | |
| Blue sclerae, as in the eyes of the girl above, are a classic non-pathognomonic sign of OI. | |
| Pronunciation | |
| Specialty | Pediatrics, medical genetics, orthopedics |
| Symptoms | Bones that break easily, blue tinge to the whites of the eye, short height, loose joints, hearing loss[2][6] |
| Onset | Birth[6] |
| Duration | Long term[6] |
| Causes | Genetic (autosomal dominant or de novo mutation)[2] |
| Diagnostic method | Based on symptoms, DNA testing[6] |
| Prevention | Pre-implantation genetic diagnosis |
| Management | Healthy lifestyle (exercise, no smoking), metal rods through the long bones[6] |
| Medication | Bisphosphonates[7] |
| Prognosis | Depends on the type[6] |
| Frequency | 1 in 10,000–20,000 people[2] |
https://en.wikipedia.org/wiki/Osteogenesis_imperfecta
Bisphosphonates are a class of drugs that prevent the loss of bone density, used to treat osteoporosis and similar diseases. They are the most commonly prescribed drugs used to treat osteoporosis.[1] They are called bisphosphonates because they have two phosphonate(PO(OH)
2) groups. They are thus also called diphosphonates (bis- or di- + phosphonate).
Evidence shows that they reduce the risk of fracture in post-menopausal women with osteoporosis.[2][3][4][5][6]
Bone tissue undergoes constant remodeling and is kept in balance (homeostasis) by osteoblasts creating bone and osteoclasts destroying bone. Bisphosphonates inhibit the digestion of bone by encouraging osteoclasts to undergo apoptosis, or cell death, thereby slowing bone loss.[7]
The uses of bisphosphonates include the prevention and treatment of osteoporosis, Paget's disease of bone, bone metastasis (with or without hypercalcemia), multiple myeloma, primary hyperparathyroidism, osteogenesis imperfecta, fibrous dysplasia, and other conditions that exhibit bone fragility.
https://en.wikipedia.org/wiki/Bisphosphonate


No comments:
Post a Comment