| Amfonelic acid | ND | ND | 207 | DRI | Stimulant |
| Amineptine*[8][9] | >100,000 (rat) | 10,000 (rat) | 1,000–1,400 (rat) | DRI | Stimulant |
| Amphetamine | >100,000 | ND | ND | NDRA | Stimulant |
| D-Amphetamine | >100,000 | 530 | 2,900 | NDRA | Stimulant |
| L-Amphetamine | >100,000 | ND | ND | NRA | Stimulant |
| Bupropion | 9,100 | 52,000 | 520 | NDRI | Stimulant |
| Cocaine[12] | 313±17 (IC50) | 292±34 (IC50) | 211±19 (IC50) | SNDRI | Stimulant |
| Methamphetamine | >100,000 | ND | ND | NDRA | Stimulant |
| D-Methamphetamine | >100,000 | 660 | 2,800 | NDRA | Stimulant |
| Cocaethylene[11] | 3,878 | >10,000 | 555 | SDRI | Stimulant |
| Sibutramine[13] | 298–2,800 | 350–5,451 | 943–1,200 | SNDRI | SNRI |
https://en.wikipedia.org/wiki/Monoamine_reuptake_inhibitor

Component
Phosphoric acid hydrogen
hydrogen proton
light particle reactions (anti heavy particle collection; anchors, chains, transpirations, transmutations)
nicotine
cyanide
phosphorous
arsenic
radiation
acid, anhydrous
graphite/graphene
cyaphide, phosphor
scale of particle (proton or hydrogen)
cyanide, cyphos, arsphentermine, diphenidine,
phosphine, phosphoric acid,
Articulates
Non bond; weak interactions; long bond (extra specificity, regenerative, cascade capability, etc.); circular capacity; spirality; zero integration; scale reduction for re-zero or zero as established at micro (variant zero considerations/literature/perspective/etc.); etc.. Vaccume forcing, fissors, etc..
Eliminate
Double bond, ketone, sugars, fats, proteins, agglomerates, etc.. rings, ringulants, large long chain polymers, epoxide cascade, expxy cascade, epox/lipod/lipification/palmization/palming/chole palm/etc., fructose, scents (rate & holes), oxygen electron OD, Oxygen hydrogen assemblies fractional second (ion stream triggers, etc..), complexes very large very complex, hidden esters/etc. (multilevel mixtures), oxygen nested chunks, bombs, nitrogen, sulfur, bacteria viands, liquification/mineralization/crystallization inducing, precipitants, antipsychotic model of flawed drug design, etc..
Physiology Models for human design HENG
Cachexia
Stephanie Anton Bettey Frankenstein
Catabolism
NADP Phosphor Adenine or Sub ATP Nicotine propylene glycol glycol proton acid mal erythritol cellulose (gum at rate channel stream) caffiene
dry coherent gas model (dust form, light transitions permeation) solid state perseverance with de-agglomeration, anti-crystallization/liquefication/solification/gellification/palmitoylation/myristoylation/etc., etc..
Note outcome - high stability endurance, power, energy, stable steady state. naps, eats very little anything, drinks very very little black coffee. drinks almost nothing. retains memory function, intelligence function, beauty function, health function.
Phosphorus pathways, adenine nicotine (NAD NADPH, ADP ATP), (possibly glycolysis), etc..
Anhydrous, DNA scale reduction light imaging time, weak molecular force time/conditions/constraints/confounds, extra-discipline model of concept/conceptualization of transmutation-alternate living state/form/etc., etc..
cytoskeleton, chondrite, carbonacious chondrite, tubulin, ADP ribosylation, point particle photon cascade time/etc., cartiledge skeletos, cartilage, filamenting filament, filamentaceous, regression analysis/regressatory/transpiratory processes, zero, diatom, matrix, junction, matricing, matrixing, settle points/collapse points, fractures, tension lines, tensors and current (impedance, capacitance, resistance, conductance, etc.), dissipative system quantum decoherence decoupling optics, zero dipole moment, pyroelectro, piezo, pyrophosphate, pyrophosos, circular dichromism, dipolar cycloaddition, membrane biology, oxidative phosphorylation, biphosphoglycerate, propionic acid chlorin pyrrole, g- factor, dirac delta, delta, alpha, etc.. muon, peon, etc.. point particle supermassive core light imager, bigger/heavier/etc. than earth very tiny. skip fructose step glyco. cosmic microwave background, earth energy standard, cyolysis before transform, membranizstion cartilidgeization phosphorylation-phosphitization, etc.. time crystal from any supermatter, optics, etc.. master preon, baby preons, forming preon soup, etc.. coovules, coovie, covid (master/adult - covid, baby - coovie, preformation/assembly components - coovules in medium [or missing/escaped part time transforming part time present part time]).
https://en.wikipedia.org/wiki/Preon
https://en.wikipedia.org/wiki/Pion
In particle physics, preons are point particles, conceived of as sub-components of quarks and leptons. The word was coined by Jogesh Pati and Abdus Salam, in 1974.
https://en.wikipedia.org/wiki/Preon
The neutrinos comprise the other three leptons, and for each neutrino there is a corresponding member from the other set of three leptons.
https://en.wikipedia.org/wiki/Preon
https://en.wikipedia.org/wiki/Neutrino
https://en.wikipedia.org/wiki/Graviton
https://en.wikipedia.org/wiki/Antiparticle
https://en.wikipedia.org/wiki/Higgs_boson
https://en.wikipedia.org/wiki/Neutroniumhttps://en.wikipedia.org/wiki/Electron_degeneracy_pressure
https://en.wikipedia.org/wiki/Strange_matter
https://en.wikipedia.org/wiki/Glycolysis
https://en.wikipedia.org/wiki/1,3-Bisphosphoglyceric_acid
https://en.wikipedia.org/wiki/Glycerol_phosphate_shuttle
https://en.wikipedia.org/wiki/Propionic_anhydride
https://en.wikipedia.org/wiki/Cellulose
https://en.wikipedia.org/wiki/Oxidative_phosphorylation
https://en.wikipedia.org/wiki/Glycerol_phosphate_shuttle
https://en.wikipedia.org/wiki/Virtual_particle
https://en.wikipedia.org/wiki/Antiparticle
https://en.wikipedia.org/w/index.php?title=Virtual_photon&redirect=no
https://en.wikipedia.org/wiki/Photon
https://en.wikipedia.org/wiki/Phonon
https://en.wikipedia.org/wiki/Superfluidity
https://en.wikipedia.org/wiki/Supersolid
https://en.wikipedia.org/wiki/Time_crystal
https://en.wikipedia.org/wiki/Adenosine_triphosphate
https://en.wikipedia.org/wiki/Polyphosphate
https://en.wikipedia.org/wiki/Nucleoside_triphosphate
https://en.wikipedia.org/wiki/Adenosine_monophosphate
https://en.wikipedia.org/wiki/Glycerol_phosphate_shuttle
https://en.wikipedia.org/wiki/Category:Cellular_respiration
https://en.wikipedia.org/wiki/Exergonic_process
https://en.wikipedia.org/wiki/Redox
https://en.wikipedia.org/wiki/Hexokinase
https://en.wikipedia.org/wiki/Phosphate#Biochemistry_of_phosphates
https://en.wikipedia.org/wiki/Carboxylic_acid
https://en.wikipedia.org/wiki/Category:Photosynthesis
https://en.wikipedia.org/wiki/Category:Uncoupling_agents
https://en.wikipedia.org/wiki/Arc_system
https://en.wikipedia.org/wiki/Anaerobic_respiration
https://en.wikipedia.org/wiki/Anaplerotic_reactions
https://en.wikipedia.org/wiki/Malate-aspartate_shuttle
https://en.wikipedia.org/wiki/Mitochondrial_shuttle
https://en.wikipedia.org/wiki/Beta_oxidation
https://en.wikipedia.org/wiki/Peroxisome
https://en.wikipedia.org/wiki/NAD%2B_Five-prime_cap
https://en.wikipedia.org/wiki/Oxygen_reduction_reaction
https://en.wikipedia.org/wiki/Nicotinamide_adenine_dinucleotide
https://en.wikipedia.org/wiki/Citric_acid_cycle
https://en.wikipedia.org/wiki/Pyruvate_decarboxylation
https://en.wikipedia.org/wiki/Malic_acid
https://en.wikipedia.org/wiki/Formate_dehydrogenase
https://en.wikipedia.org/wiki/Dissimilatory_nitrate_reduction_to_ammonium
https://en.wikipedia.org/wiki/Electrochemical_gradient
https://en.wikipedia.org/wiki/Citric_acid_cycle
https://en.wikipedia.org/wiki/Electron_transport_chain
https://en.wikipedia.org/wiki/Submitochondrial_particle
https://en.wikipedia.org/wiki/Uncoupling_protein
https://en.wikipedia.org/wiki/Glycerol_phosphate_shuttle
https://en.wikipedia.org/wiki/Dental_Materials
https://en.wikipedia.org/wiki/Orbital_blowout_fracture
https://en.wikipedia.org/wiki/Periodontal_surgery
https://en.wikipedia.org/wiki/Scoliosis
https://en.wikipedia.org/wiki/Neurapraxia
https://en.wikipedia.org/wiki/Blood_vessel_disorder
https://en.wikipedia.org/wiki/Congenital_amputation
https://en.wikipedia.org/wiki/Denervation
https://en.wikipedia.org/wiki/List_of_instruments_used_in_ophthalmology
https://en.wikipedia.org/wiki/Cricoid_cartilage
https://en.wikipedia.org/wiki/Beta-Hydroxy_beta-methylbutyric_acid
https://en.wikipedia.org/wiki/Cachexia
Concept
Zero
Scalability
Anchors (particle/field/vaccume/universe standard/scale and rate or speed/conditions/etc.) [measure point 1]
Considerations
Autoregeneration
Autocatalyst
cascade
critical fracture - disintegration - fission
Calibration extent, autocalibration extent, etc..
Environment
Time
Stable provisions assumption (variant provisions unless distant repair initiation capability/assistance/etc.)
Conditions constraints confounds etc..
Zero setters
Warp, Field, Plane, Angle, Optics, Mirror, Transmutation Nuclear
Spalling, Chipping, Holeing, Vaccume agglomerates, Projectiles, channels/streams/beams/rays/waves/frequencies/EMR/radiation/momentum/circular/angular momentum/velocity/speed/inertia/force/power/current/incidence/energy/etc..
Factors to energy transforms for shielding
Salt, Liquid, Solid, Gas, Plasma. Interstate, Transforms, Black Matter, Non-measurable, unknown, disguised, rates, scales, materials, energy, etc.. Salt crystal, mineralization liquefication functions of earth, earth ideas, universe ideas. alternate planing, plane. calibrators, calibrations.
helium 3 fermi alpha meta trihydrocat oxygen triangle cascade breaking point (fracture point for bond formation attempt)
neutronium
Neutronium (sometimes shortened to neutrium,[1] also referred to as neutrite[2]) is a hypothetical substance composed purely of neutrons. The word was coined by scientist Andreas von Antropoff in 1926 (before the 1932 discovery of the neutron) for the hypothetical "element of atomic number zero" (with zero protons in its nucleus) that he placed at the head of the periodic table (denoted by dash, no element symbol).[3][4] However, the meaning of the term has changed over time, and from the last half of the 20th century onward it has been also used to refer to extremely dense substances resembling the neutron-degenerate matter theorized to exist in the cores of neutron stars; hereinafter "degenerate neutronium" will refer to this.
Science fiction and popular literature have used the term "neutronium" to refer to an imaginary highly dense phase of matter composed primarily of neutrons, with properties useful to the story.
https://en.wikipedia.org/wiki/Neutronium
Direct restorative materials[edit]
Direct restorations are ones which are placed directly into a cavity on a tooth, and shaped to fit. The chemistry of the setting reaction for direct restorative materials is designed to be more biologically compatible. Heat and byproducts generated cannot damage the tooth or patient, since the reaction needs to take place while in contact with the tooth during restoration. This ultimately limits the strength of the materials, since harder materials need more energy to manipulate. The type of filling (restorative) material used has a minor effect on how long they last. The majority of clinical studies indicate the annual failure rates (AFRs) are between 1% and 3% with tooth colored fillings on back teeth. Note that root canaled (endodontically) treated teeth have AFR's between 2% and 12%. The main reasons for failure are cavities that occur around the filling and fracture of the real tooth. These are related to personal cavity risk and factors like grinding teeth (bruxism).[15]
Amalgam[edit]
Amalgam is a metallic filling material composed from a mixture of mercury (from 43% to 54%) and powdered alloy made mostly of silver, tin, zinc and copper, commonly called the amalgam alloy.[16] Amalgam does not adhere to tooth structure without the aid of cements or use of techniques which lock in the filling, using the same principles as a dovetail joint.
Amalgam is still used extensively in many parts of the world because of its cost effectiveness, superior strength and longevity. However, the metallic colour is not aesthetically pleasing and tooth coloured alternatives are continually emerging with increasingly comparable properties. Due to the known toxicity of the element mercury, there is some controversy about the use of amalgams. The Swedish government banned the use of mercury amalgam in June 2009.[17] Research has shown that, while amalgam use is controversial and may increase mercury levels in the human body, these levels are below safety threshold levels established by the WHO and the EPA. However, there are certain subpopulations who, due to inherited genetic variabilities, exhibit sensitivity to mercury levels lower than these threshold levels. These particular individuals may experience adverse effects caused by amalgam restoration. These include neural defects, mainly caused by impaired neurotransmitter processing.[18]
Composite resin[edit]
Composite resin fillings (also called white fillings) are a mixture of powdered glass and plastic resin, and can be made to resemble the appearance of the natural tooth. Although cosmetically superior to amalgam fillings, composite resin fillings are usually more expensive. Bis-GMA based resins contain Bisphenol A, a known endocrine disrupter chemical, and may contribute to the development of breast cancer. However, it has been demonstrated that the extremely low levels of bis-GMA released by composite restorations do not cause a significant increase in markers of renal injury, when compared to amalgam restorations. That is, there is no added risk of renal or endocrine injury in choosing composite restorations over amalgams.[18] PEX-based materials do not contain Bisphenol A and are the least cytotoxic material available.
Most modern composite resins are light-cured photopolymers, meaning that they harden with light exposure. They can then be polished to achieve maximum aesthetic results. Composite resins experience a very small amount of shrinkage upon curing, causing the material to pull away from the walls of the cavity preparation. This makes the tooth slightly more vulnerable to microleakage and recurrent decay. Microleakage can be minimized or eliminated by utilizing proper handling techniques and appropriate material selection.
In some circumstances, less tooth structure can be removed compared to preparation for other dental materials such as amalgam and many of the indirect methods of restoration. This is because composite resins bind to enamel (and dentin too, although not as well) via a micromechanical bond. As conservation of tooth structure is a key ingredient in tooth preservation, many dentists prefer placing materials like composite instead of amalgam fillings whenever possible.
Generally, composite fillings are used to fill a carious lesion involving highly visible areas (such as the central incisors or any other teeth that can be seen when smiling) or when conservation of tooth structure is a top priority.
The bond of composite resin to tooth, is especially affected by moisture contamination and cleanliness of the prepared surface. Other materials can be selected when restoring teeth where moisture control techniques are not effective.
Glass ionomer cement[edit]
The concept of using "smart" materials in dentistry has attracted a lot of attention in recent years. Conventional glass-ionomer (GI) cements have a large number of applications in dentistry. They are biocompatible with the dental pulp to some extent. Clinically, this material was initially used as a biomaterial to replace the lost osseous tissues in the human body.
These fillings are a mixture of glass and an organic acid. Although they are tooth-colored, glass ionomers vary in translucency. Although glass ionomers can be used to achieve an aesthetic result, their aesthetic potential does not measure up to that provided by composite resins.
The cavity preparation of a glass ionomer filling is the same as a composite resin. However, one of the advantages of GI compared to other restorative materials is that they can be placed in cavities without any need for bonding agents (4).
Conventional glass ionomers are chemically set via an acid-base reaction. Upon mixing of the material components, there is no light cure needed to harden the material once placed in the cavity preparation. After the initial set, glass ionomers still need time to fully set and harden.
Advantages:
- Glass ionomer can be placed in cavities without any need for bonding agents .
- They are not subject to shrinkage and microleakage, as the bonding mechanism is an acid-base reaction and not a polymerization reaction.(GICs do not undergo great dimensional changes in a moist environment in response to heat or cold and it appears heating results only in water movement within the structure of the material. These exhibit shrinkage in a dry environment at temperature higher than 50C, which is similar to the behavior of dentin.
- Glass ionomers contain and release fluoride, which is important to preventing carious lesions. Furthermore, as glass ionomers release their fluoride, they can be "recharged" by the use of fluoride-containing toothpaste. Hence, they can be used as a treatment modality for patients who are at high risk for caries. Newer formulations of glass ionomers that contain light-cured resins can achieve a greater aesthetic result, but do not release fluoride as well as conventional glass ionomers.
Disadvantages:
The most important disadvantage is lack of adequate strength and toughness. In an attempt to improve the mechanical properties of the conventional GI, resin-modified ionomers have been marketed. GICs are usually weak after setting and are not stable in water; however, they become stronger with the progression of reactions and become more resistant to moisture. New generations: The aim is tissue regeneration and use of biomaterial in the form of a powder or solution is to induce local tissue repair. These bioactive materials release chemical agents in the form of dissolved ions or growth factors such as bone morphogenic protein, which stimulates activate cells.
Glass ionomers are about as expensive as composite resin. The fillings do not wear as well as composite resin fillings. Still, they are generally considered good materials to use for root caries and for sealants.
Resin modified glass-ionomer cement (RMGIC)[edit]
A combination of glass-ionomer and composite resin, these fillings are a mixture of glass, an organic acid, and resin polymer that harden when light cured (the light activates a catalyst in the cement that causes it to cure in seconds). The cost is similar to composite resin. It holds up better than glass ionomer, but not as well as composite resin, and is not recommended for biting surfaces of adult teeth,[19] or when control of moisture cannot be achieved.[20][21]
Generally, resin modified glass-ionomer cements can achieve a better aesthetic result than conventional glass ionomers, but not as good as pure composites. It has its own setting reaction.
Compomers[edit]
[22] Another combination of composite resin and glass ionomer technology, with focus lying towards the composite resin end of the spectrum. Compomers are essentially made up of filler, dimethacrylate monomer, difunctional resin, photo-activator and initiator, and hydrophilic monomers. The primary reason of the addition of filler is to decrease the proportion of resin and increase the mechanical strength besides improving the material's appearance.
Although compomers have better mechanical and aesthetic properties than RMGIC, they have few disadvantages which limit their applications.
- Compomers have weaker wear properties.
- Compomers are not adhesive, therefore they require bonding materials. Compomers themselves cannot adhere to the tooth tissue due to the presence of resin which can make it shrink on polymerisation. As a result, any bonding attempted will be disrupted at this stage.
- Compomers release fluoride at low level, so they cannot act as a fluoride reservoir.
- Compomers have high staining susceptibility. Uptake of oral fluid causes them to show staining soon after placement.
Due to its relatively weaker mechanical properties, Compomers are unfit for stress-bearing restorations but can be used in the deciduous dentition where lower loads are anticipated.
Cermets[edit]
Dental cermets, also known as silver cermets, were created to improve the wear resistance and hardness of glass ionomer cements(mentioned above) through the addition of silver. While the incorporation of silver achieved this, cermets have poorer aesthetics, appearing metallic rather than white. Cermets also have a similar compressive strength, flexural strength, and solubility as glass ionomer cements, some of the main limiting factors for both materials. Clinical studies have shown cermets perform poorly. All these disadvantages led to the decline in the use of this restorative material.[23]
Below is a summary of the advantages and disadvantages of dental cermets.[23]
Advantages:
- Radio-opaque - this helps with identification of secondary caries when future radiographs are taken as there will be a greater contrast between the cermet and tooth tissue on the radiographic image
- Adheres directly to tooth tissue
- Higher wear resistance than glass ionomer cements (GICs)
- Greater hardness than GICs
Disadvantages:
- Low compressive strength
- Low flexural strength
- Soluble
- Poor aesthetics
- Poorer fluoride release than GICs
- Poor clinical performance
Deep plane period (1980–1991)[edit]
Tessier, who had his background in the craniofacial surgery, made the step to a subperiosteal dissection via a coronal incision.[8] In 1979, Tessier demonstrated that the subperiosteal undermining of the superior and lateral orbital rims allowed the elevation of the soft tissue and eyebrows with better results than the classic face-lifting. The objective was to elevate the soft tissue over the underlying skeleton to re-establish the patient's youthful appearance.
https://en.wikipedia.org/wiki/Rhytidectomy
Subperiosteal dissection via a coronal incision
Subperiosteal facelift[edit]
The subperiosteal facelift technique is done by vertically lifting the soft tissues of the face, completely separating it from the underlying facial bones and elevating it to a more esthetically pleasing position, correcting deep nasolabial folds and sagging cheeks. The technique is often combined with standard techniques, which provide a long-lasting rejuvenation of the face and is done in all age groups. The difference between this and other lifts is that the subperiosteal facelift has a longer period of facial swelling after the procedure.
| Facelift: Generally relevant anatomy | |
|---|---|
 Head nerves | |
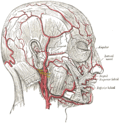 Head arteries | |
| Details | |
| Artery | Facial artery, Temporal artery, Arteria supratrochlearis, Arteria infraorbitalis |
| Vein | Temporal vein |
| Nerve | Greater auricular nerve, Facial nerve, Mental nerve |
| Identifiers | |
| MeSH | D015361 |
| Anatomical terminology | |
https://en.wikipedia.org/wiki/Rhytidectomy#Subperiosteal_facelift
https://en.wikipedia.org/wiki/Facial_nerve
https://en.wikipedia.org/wiki/Facial_artery
https://en.wikipedia.org/wiki/Superficial_temporal_artery
https://en.wikipedia.org/wiki/Superficial_temporal_vein
Liquid nitrogen[edit]
A common method of freezing lesions is by using liquid nitrogen as the cryogen. The liquid nitrogen may be applied to lesions using a variety of methods; such as dipping a cotton or synthetic material tipped applicator in liquid nitrogen and then directly applying the cryogen onto the lesion.[3] The liquid nitrogen can also be sprayed onto the lesion using a spray canister. The spray canister may utilize a variety of nozzles for different spray patterns.[3] A cryoprobe, which is a metal applicator that has been cooled using liquid nitrogen, can also be directly applied onto lesions.[3]
Carbon dioxide[edit]
Carbon dioxide is also available as a spray and is used to treat a variety of benign spots. Less frequently, doctors use carbon dioxide "snow" formed into a cylinder or mixed with acetone to form a slush that is applied directly to the treated tissue.
Argon[edit]
Recent advances in technology have allowed for the use of argon gas to drive ice formation using a principle known as the Joule-Thomson effect. This gives physicians excellent control of the ice and minimizes complications using ultra-thin 17 gauge cryoneedles.
Freeze sprays[edit]
A mixture of dimethyl ether and propane is used in some "freeze spray" preparations such as Dr. Scholl's Freeze Away. The mixture is stored in an aerosol spray type container at room temperature and drops to −41 °C (−42 °F) when dispensed. The mixture is often dispensed into a straw with a cotton-tipped swab. Similar products may use tetrafluoroethane or other substances.
https://en.wikipedia.org/wiki/Cryosurgery
Nerve injury classification[edit]
Classification of nerve damage was well-defined by Sir Herbert Seddon and Sunderland in a system that remains in use.[6] The adjacent table details the forms (neurapraxia, axonotmesis and neurotmesis) and degrees of nerve injury that occur as a result of exposure to various temperatures.
Cryoneurolysis treatments that use nitrous oxide (boiling point of -88.5 °C) as the coolant fall in the range of an axonotmesis injury, or 2nd degree injury, according to the Sunderland classification system. Treatments of the nerve in this temperature range are reversible. Nerves treated in this temperature range experience a disruption of the axon, with Wallerian degeneration occurring distal to the site of injury.[7] The axon and myelin sheathare affected, but all of the connective tissues (endoneurium, perineurium, and epineurium) remain intact.[8] Following Wallerian degeneration, the axon regenerates along the original nerve path at a rate of approximately 1–2 mm per day.[9][10][11][12]
Cryoneurolysis differs from cryoablation in that cryoablation treatments utilize liquid nitrogen (boiling point of -195.8 °C) as the coolant, and therefore, fall into the range of a neurotmesis injury, or 3rd degree injury according to the Sunderland classification. Treatments of the nerve in this temperature range are irreversible. Nerves treated in this temperature range experience a disruption of both the axon and the endoneurium connective tissue layer.[13][14]
https://en.wikipedia.org/wiki/Cryoneurolysis
| Nerve injury | |
|---|---|
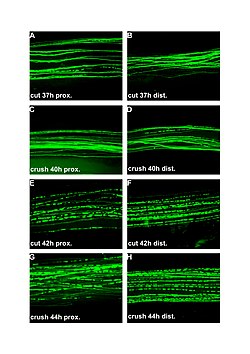 | |
| Fluorescent micrographs (100x) of Wallerian degeneration in cut and crushed peripheral nerves. Left column is proximal to the injury, right is distal. A and B: 37 hours post cut. C and D: 40 hours post crush. E and F: 42 hours post cut. G and H: 44 hours post crush. | |
| Specialty | Neurology |
https://en.wikipedia.org/wiki/Wallerian_degeneration
Schwann cells or neurolemmocytes (named after German physiologist Theodor Schwann) are the principal glia of the peripheral nervous system (PNS). Glial cells function to support neurons and in the PNS, also include satellite cells, olfactory ensheathing cells, enteric glia and glia that reside at sensory nerve endings, such as the Pacinian corpuscle. The two types of Schwann cells are myelinating and nonmyelinating.[1] Myelinating Schwann cells wrap around axons of motor and sensory neurons to form the myelin sheath. The Schwann cell promoter is present in the downstream region of the human dystrophin gene that gives shortened transcriptthat are again synthesized in a tissue-specific manner.
During the development of the PNS, the regulatory mechanisms of myelination are controlled by feedforward interaction of specific genes, influencing transcriptional cascades and shaping the morphology of the myelinated nerve fibers.[2]
Schwann cells are involved in many important aspects of peripheral nerve biology—the conduction of nervous impulses along axons, nerve development and regeneration, trophic support for neurons, production of the nerve extracellular matrix, modulation of neuromuscular synaptic activity, and presentation of antigens to T-lymphocytes.
Charcot–Marie–Tooth disease, Guillain–Barré syndrome (acute inflammatory demyelinating polyradiculopathy type), schwannomatosis, chronic inflammatory demyelinating polyneuropathy, and leprosy are all neuropathies involving Schwann cells.
https://en.wikipedia.org/wiki/Schwann_cell
Chronic inflammatory demyelinating polyneuropathy is an acquired immune-mediated inflammatory disorder of the peripheral nervous systemcharacterized by progressive weakness and impaired sensory function in the legs and arms.[1] The disorder is sometimes called chronic relapsing polyneuropathy (CRP) or chronic inflammatory demyelinating polyradiculoneuropathy (because it involves the nerve roots).[2] CIDP is closely related to Guillain–Barré syndrome and it is considered the chroniccounterpart of that acute disease.[3] Its symptoms are also similar to progressive inflammatory neuropathy.
https://en.wikipedia.org/wiki/Chronic_inflammatory_demyelinating_polyneuropathy
Dystrophin is a rod-shaped cytoplasmic protein, and a vital part of a protein complex that connects the cytoskeleton of a muscle fiber to the surrounding extracellular matrix through the cell membrane. This complex is variously known as the costamere or the dystrophin-associated protein complex(DAPC). Many muscle proteins, such as α-dystrobrevin, syncoilin, synemin, sarcoglycan, dystroglycan, and sarcospan, colocalize with dystrophin at the costamere.
The DMD gene, encoding the dystrophin protein, is one of the longest human genes known, covering 2.3 megabases (0.08% of the human genome) at locus Xp21. The primary transcript in muscle measures about 2,100 kilobases and takes 16 hours to transcribe;[5] the mature mRNAmeasures 14.0 kilobases.[6] The 79-exon muscle transcript[7] codes for a protein of 3685 amino acid residues.[8]
Spontaneous or inherited mutations in the dystrophin gene can cause different forms of muscular dystrophy, a disease characterized by progressive muscular wasting. The most common of these disorders caused by genetic defects in dystrophin is Duchenne muscular dystrophy.
https://en.wikipedia.org/wiki/Dystrophin
Schwannomatosis is an extremely rare genetic disorder closely related to the more-common disorder neurofibromatosis (NF). Originally described in Japanese patients,[1] it consists of multiple cutaneous schwannomas, central nervous system tumors, and other neurological complications, excluding hallmark signs of NF. The exact frequency of schwannomatosis cases is unknown, although some populations have noted frequencies as few as 1 case per 1.7 million people.[2]
Schwannomas are mostly benign tumors that commonly occur in individuals with NF2 and schwannomatosis (sometimes called neurofibromatosis type III). Schwann cells are glial cells that myelinate the axons of nerve cells. Myelin is a lipid covering that speeds the conduction of action potentials. When Schwann cells proliferate out of control in an encapsulation it is called a schwannoma. Although schwannomas are benign they become detrimental when the growing tumor compresses the nerve. Schwannomas on sensory nerve axons cause chronic severe pain. Treatment options for schwannomas are to surgically remove them, have radiation, cyberknife or Intracapsular Enucleation. Previous designations for schwannomas include neurinoma and neurilemmoma.[3]
https://en.wikipedia.org/wiki/Schwannomatosis
Schwann cells are a variety of glial cells that keep peripheral nerve fibres (both myelinated and unmyelinated) alive. In myelinated axons, Schwann cells form the myelin sheath. The sheath is not continuous. Individual myelinating Schwann cells cover about 1 mm of an axon[3]—equating to about 1000 Schwann cells along a 1-m length of the axon. The gaps between adjacent Schwann cells are called nodes of Ranvier.
9-O-Acetyl GD3 ganglioside is an acetylated glycolipid which is found in the cell membranes of many types of vertebrate cells. During peripheral nerve regeneration, 9-O-acetyl GD3 is expressed by Schwann cells.[4]
The vertebrate nervous system relies on the myelin sheath for insulation and as a method of decreasing membrane capacitance in the axon. The action potential jumps from node to node, in a process called saltatory conduction, which can increase conductionvelocity up to 10 times, without an increase in axonal diameter. In this sense, Schwann cells are the PNS's analogues of the central nervous system's oligodendrocytes. However, unlike oligodendrocytes, each myelinating Schwann cell provides insulation to only one axon (see image). This arrangement permits saltatory conduction of action potentials with repropagation at the nodes of Ranvier. In this way, myelination greatly increases speed of conduction and saves energy.[5]
Nonmyelinating Schwann cells are involved in maintenance of axons and are crucial for neuronal survival. Some group around smaller axons (External image here) and form Remak bundles.
Myelinating Schwann cells begin to form the myelin sheath in mammals during fetal development and work by spiraling around the axon, sometimes with as many as 100 revolutions. A well-developed Schwann cell is shaped like a rolled-up sheet of paper, with layers of myelin between each coil. The inner layers of the wrapping, which are predominantly membrane material, form the myelin sheath, while the outermost layer of nucleated cytoplasm forms the neurilemma. Only a small volume of residual cytoplasm allows communication between the inner and outer layers. This is seen histologically as the Schmidt-Lantermann incisure.
Regeneration[edit]
Schwann cells are known for their roles in supporting nerve regeneration.[6] Nerves in the PNS consist of many axons myelinated by Schwann cells. If damage occurs to a nerve, the Schwann cells aid in digestion of its axons (phagocytosis). Following this process, the Schwann cells can guide regeneration by forming a type of tunnel that leads toward the target neurons. This tunnel is known as band of Büngner, a guidance track for the regenerating axons, which behaves like an endoneural tube. The stump of the damaged axon is able to sprout, and those sprouts that grow through the Schwann-cell “tunnel” do so at the rate around 1 mm/day in good conditions. The rate of regeneration decreases with time. Successful axons can, therefore, reconnect with the muscles or organs they previously controlled with the help of Schwann cells, but specificity is not maintained and errors are frequent, especially when long distances are involved.[7] Because of their ability to impact regeneration of axons, Schwann cells have been connected to preferential motor reinnervation, as well. If Schwann cells are prevented from associating with axons, the axons die. Regenerating axons will not reach any target unless Schwann cells are there to support them and guide them. They have been shown to be in advance of the growth cones.
Schwann cells are essential for the maintenance of healthy axons. They produce a variety of factors, including neurotrophins, and also transfer essential molecules across to axons.
Genetics[edit]
Schwann cell formation[edit]
Sox10[edit]
SOX10 is a transcription factor active during embryonic development and abundant evidence indicates that it is essential for the generation of glial lineages from trunk crest cells.[8][9] When SOX10 is inactivated in mice, satellite glia and Schwann cell precursors fail to develop, though neurons are generated normally without issue.[8] In the absence of SOX10, neural crest cells survive and are free to generate neurons, but glial specification is blocked.[9] SOX10 might influence early glial precursors to respond to neuregulin 1[8] (see below).
Neuregulin 1[edit]
Neuregulin 1 (NRG1) acts in a number of ways to both promote the formation and ensure the survival of immature Schwann cells.[10] During embryonic development, NRG1 inhibits the formation of neurons from neural crest cells, instead contributing to neural crest cells being led down a path to gliogenesis. NRG1 signaling is not, however, required for glial differentiation from the neural crest.[11]
NRG1 plays important roles in the development of neural crest derivatives. It is required for neural crest cells to migrate past the site of dorsal root ganglia to find the ventral regions of sympathetic gangliogenesis.[12] It is also an essential axon-derived survival factor and a mitogen for Schwann cell precursors.[13] It is found in the dorsal root ganglion and motor neurons at the point in time that Schwann cell precursors begin to populate spinal nerves and therefore influences Schwann cell survival.[11] In embryonic nerves, the transmembrane III isoform likely is the primary variant of NRG1 responsible for survival signals. In mice that lack the transmembrane III isoform, Schwann cell precursors are eventually eliminated from spinal nerves.[14]
Formation of myelin sheath[edit]
P0[edit]
Myelin protein zero (P0) is a cell-adhesion molecule belonging to the immunoglobulin superfamily and is the major component of peripheral myelin, constituting over 50% of the total protein in the sheath.[15][16] P0 has been shown to be essential for the formation of compact myelin, as P0 null mutant (P0-) mice showed severely aberrant peripheral myelination.[17] Although myelination of large caliber axons was initiated in P0- mice, the resulting myelin layers were very thin and poorly compacted. Unexpectedly, P0- mice also showed degeneration of both axons and their surround myelin sheaths, suggesting that P0 plays a role in maintaining the structural integrity of both myelin formation and the axon with which it’s associated. P0- mice developed behavioral deficits around 2 weeks of age when mice began to show signs of slight trembling. Gross incoordination also arose as the animals developed, while trembling became more severe and some older mice developed convulsing behaviors. Despite the array of impaired motor behavior, no paralysis was observed in these animals. P0 is also an important gene expressed early within the Schwann cell lineage, expressed in Schwann cell precursors after differentiating from migrating neural crest cells within the developing embryo.[18]
Krox-20[edit]
Several important transcription factors are also expressed and involved at various stages in development changing the features on the Schwann cells from an immature to mature state. One indispensable transcription factor expressed during the myelination process is Krox-20. It is a general zinc-finger transcription factor and is expressed in the rhombomeres 3 and 5.
Krox-20 is considered one of the master regulators of PNS myelination and is important in driving transcription of specific structural proteins in the myelin. It has been shown to control a set of genes responsible for interfering with this feature in the axon changing it from a pro-myelinating to myelinating state.[19] In this way, in Krox-20 double knock out mice, it has been recorded that hindbrain segmentation is affected as well as myelination of Schwann cell associated axons. Indeed, in these mice, the Schwann cells are not able to perform their myelination properly as they only wrap their cytoplasmic processes one and half turn around the axon and despite the fact that they still express the early myelin marker, late myelin gene products are absent. In addition, recent studies have also proven the importance of this transcription factor in maintaining the myelination phenotype (and requires the co-expression of Sox 10) as its inactivation leads to dedifferentiation of the Schwann cells.[2]
https://en.wikipedia.org/wiki/Schwann_cell
Saltatory conduction (from the Latin saltare, to hop or leap) is the propagation of action potentials along myelinated axons from one node of Ranvier to the next node, increasing the conduction velocity of action potentials. The uninsulated nodes of Ranvier are the only places along the axon where ions are exchanged across the axon membrane, regenerating the action potential between regions of the axon that are insulated by myelin, unlike electrical conduction in a simple circuit.
https://en.wikipedia.org/wiki/Saltatory_conduction
In neurobiology, a mesaxon is a pair of parallel plasma membranes of a Schwann cell.[1][2] It marks the point of edge-to-edge contact by the Schwann cell encircling the axon.[2] A single Schwann cell of the peripheral nervous system will wrap around and support only one individual axon (then myelinated; ratio of 1:1), while the oligodendrocytes found in the central nervous system can wrap around and support 5-8 axons. Thin unmyelinated axons are often bundled, with several unmyelinated axons to a single mesaxon (and several such groups to a single Schwann cell).
The outer mesaxon (Terminologia histologica: Mesaxon externum) is the connection of the outer cell membrane to the compact myelin sheath. The inner mesaxon (Terminologia histologica: Mesaxon internum) is the connection between the myelin sheath and the inner part of the cell membrane of the Schwann cell, which is directly opposite the axolemma, i.e. the cell membrane of the nerve fibre ensheathed by the Schwann cell.
https://en.wikipedia.org/wiki/Mesaxon
Olfactory ensheathing cells (OECs), also known as olfactory ensheathing glia or olfactory ensheathing glial cells, are a type of macroglia (radial glia) found in the nervous system. They are also known as olfactory Schwann cells, because they ensheath the non-myelinated axons of olfactory neurons in a similar way to which Schwann cells ensheath non-myelinated peripheral neurons. They also share the property of assisting axonal regeneration.
OECs are capable of phagocytosing axonal debris in vivo, and in vitro they phagocytose bacteria. Olfactory glia that express the antimicrobial enzyme lysozyme(LYZ) are thought to play an important role in immunoprotection in the mucosa, where neurons are directly exposed to the external environment.
OECs have been tested successfully in experimental axonal regeneration in adult rats with traumatic spinal cord damage, and clinical trials are currently being conducted to obtain more information on spinal cord injuries and other neurodegenerative diseases.
https://en.wikipedia.org/wiki/Olfactory_ensheathing_cell
A schwannoma is a usually benign nerve sheath tumor composed of Schwann cells, which normally produce the insulating myelin sheath covering peripheral nerves.
https://en.wikipedia.org/wiki/Schwannoma
This is a list of cells in humans derived from the three embryonic germ layers – ectoderm, mesoderm, and endoderm.
Cells derived from ectoderm[edit]
Surface ectoderm[edit]
Skin[edit]
Anterior pituitary[edit]
Tooth enamel[edit]
Neural crest[edit]
Peripheral nervous system[edit]
Neuroendocrine system[edit]
Skin[edit]
Teeth[edit]
Eyes[edit]
Neural tube[edit]
Central nervous system[edit]
- Neuron
- Glia glial cells and microglial cells
- Astrocyte
- Ependymocytes
- Muller glia (retina)
- Oligodendrocyte
- Pituicyte (posterior pituitary)
Pineal gland[edit]
Cells derived from mesoderm[edit]
Paraxial mesoderm[edit]
Mesenchymal stem cell[edit]
Osteochondroprogenitor cell[edit]
- Bone (Osteoblast → Osteocyte)
- Cartilage (Chondroblast → Chondrocyte)
Myofibroblast[edit]
- Fat
- Muscle
- Other
Other[edit]
- Digestive system
Intermediate mesoderm[edit]
Renal stem cell[edit]
- Angioblast → Endothelial cell
- Mesangial cell
- Juxtaglomerular cell
- Macula densa cell
- Stromal cell → Interstitial cell → Telocytes
- Simple epithelial cell → Podocyte
- Kidney proximal tubule brush border cell
Reproductive system[edit]
Lateral plate mesoderm/hemangioblast[edit]
Hematopoietic stem cell[edit]
Circulatory system[edit]
- Endothelial progenitor cell
- Endothelial colony forming cell
- Endothelial stem cell
- Angioblast/Mesoangioblast
- Pericyte
- Mural cell
Cells derived from endoderm[edit]
Foregut[edit]
Respiratory system[edit]
Digestive system[edit]
Stomach[edit]
Intestine[edit]
Liver[edit]
Gallbladder[edit]
Exocrine component of pancreas[edit]
Islets of Langerhans[edit]
- alpha cell
- beta cell
- delta cell
- PP cell (F cell, gamma cell)
- epsilon cell
Pharyngeal pouch[edit]
- Thyroid gland
- Parathyroid gland
Hindgut/cloaca[edit]
See also[edit]
https://en.wikipedia.org/wiki/List_of_human_cell_types_derived_from_the_germ_layers
Pages in category "Gastrulation"
The following 11 pages are in this category, out of 11 total. This list may not reflect recent changes (learn more).
V
Pages in category "Germ layers"
The following 6 pages are in this category, out of 6 total. This list may not reflect recent changes (learn more).
https://en.wikipedia.org/wiki/Category:Germ_layers
Subcategories
This category has the following 11 subcategories, out of 11 total.
*
E
- Embryology of digestive system (16 P)
- Embryology of nervous system (44 P)
- Embryology of urogenital system (34 P)
F
G
N
- Nipple (19 P)
P
- Pharyngeal arches (9 P)
T
Pages in category "Embryology"
The following 173 pages are in this category, out of 173 total. This list may not reflect recent changes (learn more).
A
B
C
- Canine follicle development
- Carnegie stages
- Caudal cell mass
- Chicken as biological research model
- Chondrogenesis
- Choriogenesis
- Chorion
- Chorionic villi
- Choriovitelline placenta
- Clock and wavefront model
- Comparative embryology
- Conceptus
- Connecting stalk
- Cord lining
- Crista dividens
- Crown-rump length
- Cytotrophoblast
- Cytotrophoblastic shell
D
E
- Early stages of embryogenesis of tailless amphibians
- Ectoderm
- Embryo fossil
- Embryo loss
- Embryokine
- Embryomics
- Embryonated
- Embryonic development
- Embryonic differentiation waves
- Embryonic disc
- Embryonic stem cell
- Embryotroph
- Endoderm
- Endodermic evagination
- Enterocoely
- Environmental toxicants and fetal development
- Epiblast
- Epigenesis (biology)
- Epipharyngeal groove
- European Assisted Conception Consortium
- European Society of Human Reproduction and Embryology
- Extraembryonic membrane
F
H
I
L
M
P
S
- Schizocoely
- Secondary palate development
- Segment polarity gene
- Segmentation gene
- Septum transversum
- Sex reversal
- Shell-less chick embryo culture
- Sinus tubercle
- Somatic embryogenesis
- Somatopleuric mesenchyme
- Somite
- Somitogenesis
- Somitomere
- Spemann-Mangold organizer
- Splanchnopleuric mesenchyme
- Standard Event System
- Stigma (anatomy)
- Studies of the Fetus in the Womb
- Surface ectoderm
- Surfactant–albumin ratio
- Syncytiotrophoblast
T
V
Z

References[edit]
- Sadler, T.W (2004). Langman's Medical Embryology (Ninth ed.). Lippincott Willams & Wilkins. pp. 388–394. ISBN 0-7817-4310-9.
https://en.wikipedia.org/wiki/Nasal_placode
The pharyngeal arches, also known as visceral arches, are structures seen in the embryonic development of vertebrates that are recognisable precursors for many structures. In fish, the arches are known as the branchial arches, or gill arches.
In the human embryo, the arches are first seen during the fourth week of development. They appear as a series of outpouchings of mesoderm on both sides of the developing pharynx. The vasculature of the pharyngeal arches is known as the aortic arches.
In fish, the branchial arches support the gills.
| Pharyngeal arch | |
|---|---|
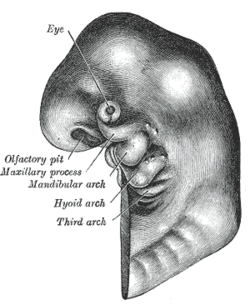 Schematic of developing human fetus with first, second and third arches labelled |
The skeletal elements and muscles are derived from mesoderm of the pharyngeal arches.
Skeletal
- malleus and incus of the middle ear
- maxilla and mandible
- spine of sphenoid bone
- sphenomandibular ligament
- palatine bone
- squamous part of temporal bone
- anterior ligament of malleus
Muscles
- muscles of mastication (chewing)
- mylohyoid muscle
- digastric muscle, anterior belly
- tensor veli palatini muscle
- tensor tympani muscle
Other
Mucous membrane and glands of the anterior two thirds of the tongue are derived from ectoderm and endoderm of the arch.
https://en.wikipedia.org/wiki/Pharyngeal_arch
https://en.wikipedia.org/wiki/Polychlorinated_biphenyl
https://en.wikipedia.org/wiki/Carbofuran
https://en.wikipedia.org/wiki/Alfatradiol/dexamethasone
https://en.wikipedia.org/wiki/Doxepin
https://en.wikipedia.org/wiki/Ethidium_bromide
https://en.wikipedia.org/wiki/Haloperidol
https://en.wikipedia.org/wiki/Haloperidol_decanoate
https://en.wikipedia.org/wiki/Hexachlorobenzene
https://en.wikipedia.org/wiki/Amantadine
https://en.wikipedia.org/wiki/Fumonisin_B1
https://en.wikipedia.org/wiki/Gossypol
https://en.wikipedia.org/wiki/Rimantadine
https://en.wikipedia.org/wiki/Doxorubicin
https://en.wikipedia.org/wiki/Paraquat
https://en.wikipedia.org/wiki/Mirex
https://en.wikipedia.org/wiki/Dichloromethane
https://en.wikipedia.org/wiki/Temozolomide
https://en.wikipedia.org/wiki/Environmental_toxicants_and_fetal_development
https://en.wikipedia.org/wiki/Lapacho
https://en.wikipedia.org/wiki/Reserpine
https://en.wikipedia.org/wiki/Echinocandin
https://en.wikipedia.org/wiki/Phthalate
https://en.wikipedia.org/wiki/Furylfuramide
https://en.wikipedia.org/wiki/Chlorpyrifos
https://en.wikipedia.org/wiki/Acrylic_acid
https://en.wikipedia.org/wiki/Acrylamide
https://en.wikipedia.org/wiki/Amaranth_(dye)
https://en.wikipedia.org/wiki/Category:Abortifacients
https://en.wikipedia.org/wiki/Toxic_abortion
https://en.wikipedia.org/wiki/Flusilazole
https://en.wikipedia.org/wiki/Lisinopril
https://en.wikipedia.org/wiki/Mebendazole
https://en.wikipedia.org/wiki/Miltefosine
https://en.wikipedia.org/wiki/N-Nitroso-N-methylurea
https://en.wikipedia.org/wiki/Oprelvekin
https://en.wikipedia.org/wiki/Oxcarbazepine
https://en.wikipedia.org/wiki/Ulipristal_acetate
The splanchnocranium (or visceral skeleton) is the portion of the cranium that is derived from pharyngeal arches. Splanchnoindicates to the gut because the face forms around the mouth, which is an end of the gut.[1] The splanchnocranium consists of cartilage and endochondral bone. In mammals, the splanchnocranium comprises the three ear ossicles (i.e., incus, malleus, and stapes), as well as the alisphenoid, the styloid process, the hyoid apparatus, and the thyroid cartilage.[2]
In other tetrapods, such as amphibians and reptiles, homologous bones to those of mammals, such as the quadrate, articular, columella, and entoglossus are part of the splanchnocranium.[2]
https://en.wikipedia.org/wiki/Splanchnocranium
Above. Dick Dale & The Del Tones - Misirlou (1963)
Pinopodes[edit]
Pinopodes are small, finger-like protrusions from the endometrium. They appear between day 19 and day 21[6] of gestational age. This corresponds to a fertilization age of approximately five to seven days, which corresponds well with the time of implantation. They only persist for two to three days.[6] The development of them is enhanced by progesterone but inhibited by estrogens.
Function in implantation[edit]
Pinopodes endocytose uterine fluid and macromolecules in it. By doing so, the volume of the uterus decreases, taking the walls closer to the embryoblast floating in it. Thus, the period of active pinocytes might also limit the implantation window.[6]
| proteins, glycoproteins and peptides secreted by the endometrial glands[6] |
| Matrix-associated: |
| Fibronectin |
| Laminin |
| Entactin |
| Type-IV collagen |
| heparan sulfate |
| Proteoglycan |
| Integrins |
Apposition[edit]
The very first, loose connection between the blastocyst and the endometrium is called the apposition.[6]
https://en.wikipedia.org/wiki/Implantation_(human_embryo)
Plasmin is an important enzyme (EC 3.4.21.7) present in blood that degrades many blood plasma proteins, including fibrin clots. The degradation of fibrin is termed fibrinolysis. In humans, the plasmin protein is encoded by the PLG gene.[5]
https://en.wikipedia.org/wiki/Plasmin
STIMULANTS CONTINUED
Renin (etymology and pronunciation), also known as an angiotensinogenase, is an aspartic protease protein and enzyme secreted by the kidneys that participates in the body's renin–angiotensin–aldosterone system (RAAS)—also known as the renin–angiotensin–aldosterone axis—that mediates the volume of extracellular fluid (blood plasma, lymph and interstitial fluid) and arterial vasoconstriction. Thus, it regulates the body's mean arterial blood pressure.
Renin is not commonly referred to as a hormone, albeit it having a receptor, the (pro)renin receptor, also known as the renin receptor and prorenin receptor (see also below),[4] as well as enzymatic activity with which it hydrolyzes angiotensinogen to angiotensin I.
https://en.wikipedia.org/wiki/Renin
The carbonic anhydrases (or carbonate dehydratases) form a family of enzymesthat catalyze the interconversion between carbon dioxide and water and the dissociated ions of carbonic acid (i.e. bicarbonate and hydrogen ions).[1] The active site of most carbonic anhydrases contains a zinc ion. They are therefore classified as metalloenzymes. The enzyme maintains acid-base balance and helps transport carbon dioxide.[2]
Carbonic anhydrase helps maintain acid–base homeostasis, regulate pH, and fluid balance. Depending on its location, the role of the enzyme changes slightly. For example, carbonic anhydrase produces acid in the stomach lining. In the kidney, the control of bicarbonate ions influences the water content of the cell. The control of bicarbonate ions also influences the water content in the eyes. Inhibitors of carbonic anhydrase are used to treat glaucoma, the excessive build up of water in the eyes. Blocking this enzyme shifts the fluid balance in the eyes of the patient to reduce fluid build up thereby relieving pressure.[2][3]
The Bohr Effect is a way to describe hemoglobin's oxygen binding affinity. The Bohr Effect was described by Christian Bohr in the year 1904, and it refers to a shift in an oxygen dissociation curve that is caused by a change in concentration of carbon dioxide or a change in the pH. Essentially an increase in carbon dioxide results in lowered blood pH which lowers oxygen-hemoglobin binding.[4] The opposite is true where a decrease in the concentration of carbon dioxide raises the blood pH which raises the rate of oxygen-hemoglobin binding. Relating the Bohr Effect to carbonic anhydrase is simple: carbonic anhydrase speeds up the reaction of carbon dioxide reacting with water to produce hydrogen ions (protons) and bicarbonate ions.
To describe equilibrium in the carbonic anhydrase reaction, Le Chatelier's principle is used. The tissues are more acidic than the lungs because carbon dioxide is produced by cellular respiration and it reacts with water in the tissues to produce the hydrogen protons. Because the carbon dioxide concentration is higher, equilibrium shifts to the right, to the bicarbonate side. The opposite is seen in the lungs where carbon dioxide is being released so its concentration is lower so equilibrium shifts to the left towards carbon dioxide to try and raise its concentration.[5]
https://en.wikipedia.org/wiki/Carbonic_anhydrase
Beta-Endorphin or β-Endorphin is an endogenous opioid neuropeptide and peptide hormone that is produced in certain neurons within the central nervous system and peripheral nervous system.[1] It is one of three endorphins that are produced in humans, the others of which include α-endorphin and γ-endorphin.[2]
The amino acid sequence is: Tyr-Gly-Gly-Phe-Met-Thr-Ser-Glu-Lys-Ser-Gln-Thr-Pro-Leu-Val-Thr-Leu-Phe-Lys-Asn-Ala-Ile-Ile-Lys-Asn-Ala-Tyr-Lys-Lys-Gly-Glu (31 amino acids).[1][3] The first 16 amino acids are identical to α-endorphin. β-Endorphin is considered to be a part of the endogenous opioid and endorphin classes of neuropeptides;[1] all of the established endogenous opioid peptides contain the same N-terminal amino acid sequence, Tyr-Gly-Gly-Phe, followed by either -Met or -Leu.[1]
Function of β-endorphin has been known to be associated with hunger, thrill, pain, maternal care, sexual behavior, and reward cognition. In the broadest sense, β-endorphin is primarily utilized in the body to reduce stress and maintain homeostasis. In behavioral research, studies have shown that β-endorphin is released via volume transmission into the ventricular system in response to a variety of stimuli, and novel stimuli in particular.[4]
https://en.wikipedia.org/wiki/Beta-Endorphin
An enkephalin is a pentapeptide involved in regulating nociception in the body. The enkephalins are termed endogenous ligands, as they are internally derived and bind to the body's opioid receptors. Discovered in 1975, two forms of enkephalin have been found, one containing leucine ("leu"), and the other containing methionine ("met"). Both are products of the proenkephalin gene.[2]
- Met-enkephalin is Tyr-Gly-Gly-Phe-Met.
- Leu-enkephalin has Tyr-Gly-Gly-Phe-Leu.
An amine oxidase is an enzyme that catalyzes the oxidative cleavage of alkylamines into aldehydes and ammonia:[1]
- RCH2NH2 + H2O + O2 RCHO + NH3 + H2O2
Amine oxidases are divided into two subfamilies based on the cofactor they contain:
| Class | Cofactor | Subclass | Enzyme Commission number | Human genes |
|---|---|---|---|---|
| Amine oxidase (formerly EC 1.4.3.6) | copper | lysyl oxidase | EC 1.4.3.13 | LOX |
| primary-amine oxidase | EC 1.4.3.21 | AOC2, AOC3 | ||
| diamine oxidase | EC 1.4.3.22 | AOC1 | ||
| Monoamine oxidase | flavin | N/A | EC 1.4.3.4 | MAOA, MAOB |
References[edit]
- ^ Mondovì B, Finazzi Agrò A (1982). "Structure and function of amine oxidase". Advances in Experimental Medicine and Biology. Advances in Experimental Medicine and Bioligy. 148: 141–53. doi:10.1007/978-1-4615-9281-5_12. ISBN 978-1-4615-9283-9. PMID 7124512.
Endorphins (contracted from "endogenous morphine"[1][2]) are endogenous opioid neuropeptides and peptide hormones in humans and other animals. They are produced and stored in the pituitary gland. The classification of molecules as endorphins is based on their pharmacological activity, as opposed to a specific chemical formulation.
The endorphin class consists of α-endorphin, β-endorphin, and γ-endorphin. All three preferentially bind to μ-opioid receptors.[3] The principal function of endorphins is to inhibit the communication of pain signals. Endorphins may also produce a feeling of euphoriavery similar to that produced by other opioids.[4]
https://en.wikipedia.org/wiki/Endorphins
The human brain is the central organ of the human nervous system, and with the spinal cord makes up the central nervous system. The brain consists of the cerebrum, the brainstem and the cerebellum. It controls most of the activities of the body, processing, integrating, and coordinating the information it receives from the sense organs, and making decisions as to the instructions sent to the rest of the body. The brain is contained in, and protected by, the skull bones of the head.
The cerebrum, the largest part of the human brain, consists of two cerebral hemispheres. Each hemisphere has an inner core composed of white matter, and an outer surface – the cerebral cortex – composed of grey matter. The cortex has an outer layer, the neocortex, and an inner allocortex. The neocortex is made up of six neuronal layers, while the allocortex has three or four. Each hemisphere is conventionally divided into four lobes – the frontal, temporal, parietal, and occipital lobes. The frontal lobe is associated with executive functions including self-control, planning, reasoning, and abstract thought, while the occipital lobe is dedicated to vision. Within each lobe, cortical areas are associated with specific functions, such as the sensory, motor and association regions. Although the left and right hemispheres are broadly similar in shape and function, some functions are associated with one side, such as language in the left and visual-spatial ability in the right. The hemispheres are connected by commissural nerve tracts, the largest being the corpus callosum.
The cerebrum is connected by the brainstem to the spinal cord. The brainstem consists of the midbrain, the pons, and the medulla oblongata. The cerebellum is connected to the brainstem by three pairs of nerve tracts called cerebellar peduncles. Within the cerebrum is the ventricular system, consisting of four interconnected ventricles in which cerebrospinal fluid is produced and circulated. Underneath the cerebral cortex are several important structures, including the thalamus, the epithalamus, the pineal gland, the hypothalamus, the pituitary gland, and the subthalamus; the limbic structures, including the amygdala and the hippocampus; the claustrum, the various nuclei of the basal ganglia; the basal forebrain structures, and the three circumventricular organs. The cells of the brain include neurons and supportive glial cells. There are more than 86 billion neurons in the brain, and a more or less equal number of other cells. Brain activity is made possible by the interconnections of neurons and their release of neurotransmitters in response to nerve impulses. Neurons connect to form neural pathways, neural circuits, and elaborate network systems. The whole circuitry is driven by the process of neurotransmission.
The brain is protected by the skull, suspended in cerebrospinal fluid, and isolated from the bloodstream by the blood–brain barrier. However, the brain is still susceptible to damage, disease, and infection. Damage can be caused by trauma, or a loss of blood supply known as a stroke. The brain is susceptible to degenerative disorders, such as Parkinson's disease, dementias including Alzheimer's disease, and multiple sclerosis. Psychiatric conditions, including schizophrenia and clinical depression, are thought to be associated with brain dysfunctions. The brain can also be the site of tumours, both benign and malignant; these mostly originate from other sites in the body.
The study of the anatomy of the brain is neuroanatomy, while the study of its function is neuroscience. Numerous techniques are used to study the brain. Specimens from other animals, which may be examined microscopically, have traditionally provided much information. Medical imaging technologies such as functional neuroimaging, and electroencephalography (EEG) recordings are important in studying the brain. The medical history of people with brain injury has provided insight into the function of each part of the brain. Brain research has evolved over time, with philosophical, experimental, and theoretical phases. An emerging phase may be to simulate brain activity.[3]
In culture, the philosophy of mind has for centuries attempted to address the question of the nature of consciousness and the mind-body problem. The pseudoscience of phrenology attempted to localise personality attributes to regions of the cortex in the 19th century. In science fiction, brain transplants are imagined in tales such as the 1942 Donovan's Brain.
https://en.wikipedia.org/wiki/Human_brain
Metalloprotein is a generic term for a protein that contains a metal ion cofactor.[1][2] A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins (out of ~20,000) contain zinc-binding protein domains[3] although there may be up to 3000 human zinc metalloproteins.[4]
https://en.wikipedia.org/wiki/Metalloprotein
Reaction[edit]
The reaction that shows the catalyzation of carbonic anhydrase in our tissues is:
- CO2 + H2O H
2CO
3 H+ + HCO−
3
The catalyzation of carbonic anhydrase in the lungs is shown by:
- H+ + HCO−
3 H
2CO
3 CO2 + H2O
The reason for the reactions being in opposite directions for the tissues and lungs is because of the different pH levels found in them. Without the carbonic anhydrase catalyst, the reaction is very slow, however with the catalyst the reaction is 107 times faster.
The reaction catalyzed by carbonic anhydrase is:
- HCO−
3 + H+ CO2 + H2O
Carbonic acid has a pKa of around 6.36 (the exact value depends on the medium), so at pH 7 a small percentage of the bicarbonate is protonated.
Carbonic anhydrase is one of the fastest enzymes, and its rate is typically limited by the diffusion rate of its substrates. Typical catalytic rates of the different forms of this enzyme ranging between 104 and 106 reactions per second.[9]
The uncatalyzed reverse reaction is relatively slow (kinetics in the 15-second range). This is why a carbonated drink does not instantly degas when opening the container; however, it will rapidly degas in the mouth when it comes in contact with carbonic anhydrase that is contained in saliva.[10]
An anhydrase is defined as an enzyme that catalyzes the removal of a water molecule from a compound, and so it is this "reverse" reaction that gives carbonic anhydrase its name, because it removes a water molecule from carbonic acid.
In the lungs carbonic anhydrase converts bicarbonate to carbon dioxide, suited for exhalation.
n chemistry, carbonic acid is a dibasic acid with the chemical formula H2CO3. The pure compound decomposes at temperatures greater than ca. −80 °C.[2]
In biochemistry, the name "carbonic acid" is often applied to aqueous solutions of carbon dioxide, which play an important role in the bicarbonate buffer system, used to maintain acid–base homeostasis.[3]
https://en.wikipedia.org/wiki/Carbonic_acid
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula C6H5OH. It is a white crystalline solid that is volatile. The molecule consists of a phenyl group (−C6H5) bonded to a hydroxy group (−OH). Mildly acidic, it requires careful handling because it can cause chemical burns.
Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 billion kg/year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds.[8] It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivativesare essential for production of polycarbonates, epoxies, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical drugs.
| Names | |
|---|---|
| Preferred IUPAC name Phenol[1] | |
| Systematic IUPAC name Benzenol | |
| Other names Carbolic acid Phenylic acid Hydroxybenzene Phenic acid |
recent in silico comparison of the gas phase acidities of the vinylogues of phenol and cyclohexanol in conformations that allow for or exclude resonance stabilization leads to the inference that about 1⁄3 of the increased acidity of phenol is attributable to inductive effects, with resonance accounting for the remaining difference.[12]
Hydrogen bonding[edit]
In carbon tetrachloride and alkane solvents phenol hydrogen bonds with a wide range of Lewis bases such as pyridine, diethyl ether, and diethyl sulfide. The enthalpies of adduct formation and the –OH IR frequency shifts accompanying adduct formation have been studied.[13] Phenol is classified as a hard acid which is compatible with the C/E ratio of the ECW model with EA = 2.27 and CA = 1.07. The relative acceptor strength of phenol toward a series of bases, versus other Lewis acids, can be illustrated by C-B plots.[14][15]
Phenoxide anion[edit]
The phenoxide anion is a strong nucleophile with a nucleophilicity comparable to the one of carbanions or tertiary amines.[16] It can react at both its oxygen or carbon sites as an ambident nucleophile (see HSAB theory). Generally, oxygen attack of phenoxide anions is kinetically favored, while carbon-attack is thermodynamically preferred (see Thermodynamic versus kinetic reaction control). Mixed oxygen/carbon attack and by this a loss of selectivity is usually observed if the reaction rate reaches diffusion control.[17]
Tautomerism[edit]
Phenol exhibits keto-enol tautomerism with its unstable keto tautomer cyclohexadienone, but only a tiny fraction of phenol exists as the keto form. The equilibrium constant for enolisation is approximately 10−13, which means only one in every ten trillion molecules is in the keto form at any moment.[18] The small amount of stabilisation gained by exchanging a C=C bond for a C=O bond is more than offset by the large destabilisation resulting from the loss of aromaticity. Phenol therefore exists essentially entirely in the enol form.[19] 4, 4' Substituted cyclohexadienone can undergo Dienone-phenol rearrangement in acid conditions and form stable 3,4‐disubstituted phenol.[20]
Phenoxides are enolates stabilised by aromaticity. Under normal circumstances, phenoxide is more reactive at the oxygen position, but the oxygen position is a "hard" nucleophile whereas the alpha-carbon positions tend to be "soft".[21]
Older methods[edit]
Early methods relied on extraction of phenol from coal derivatives or the hydrolysis of benzene derivatives.
Hydrolysis of benzenesulfonic acid[edit]
An early commercial route, developed by Bayer and Monsanto in the early 1900s, begins with the reaction of a strong base with benzenesulfonic acid. The conversion is represented by this idealized equation:[29]
- C6H5SO3H + 2 NaOH → C6H5OH + Na2SO3 + H2O
Hydrolysis of chlorobenzene[edit]
Chlorobenzene can be hydrolyzed to phenol using base (Dow process) or steam (Raschig–Hooker process):[25][26][30]
- C6H5Cl + NaOH → C6H5OH + NaCl
- C6H5Cl + H2O → C6H5OH + HCl
These methods suffer from the cost of the chlorobenzene and the need to dispose of the chloride by product.
Coal pyrolysis[edit]
Phenol is also a recoverable byproduct of coal pyrolysis.[30] In the Lummus Process, the oxidation of toluene to benzoic acid is conducted separately.
Miscellaneous methods[edit]
Phenyldiazonium salts hydrolyze to phenol. The method is of no commercial interest since the precursor is expensive.[31]
Salicylic acid decarboxylates to phenol.[32]
Uses[edit]
The major uses of phenol, consuming two thirds of its production, involve its conversion to precursors for plastics. Condensation with acetone gives bisphenol-A, a key precursor to polycarbonates and epoxide resins. Condensation of phenol, alkylphenols, or diphenols with formaldehyde gives phenolic resins, a famous example of which is Bakelite. Partial hydrogenation of phenol gives cyclohexanone, a precursor to nylon. Nonionic detergents are produced by alkylation of phenol to give the alkylphenols, e.g., nonylphenol, which are then subjected to ethoxylation.[8]
Phenol is also a versatile precursor to a large collection of drugs, most notably aspirin but also many herbicides and pharmaceutical drugs.
Phenol is a component in liquid–liquid phenol–chloroform extraction technique used in molecular biology for obtaining nucleic acidsfrom tissues or cell culture samples. Depending on the pH of the solution either DNA or RNA can be extracted.
Medical[edit]
Phenol is widely used as an antiseptic, its use was pioneered by Joseph Lister (see History section).
From the early 1900s to the 1970s it was used in the production of carbolic soap. Concentrated phenol liquids are commonly used for permanent treatment of ingrown toe and finger nails, a procedure known as a chemical matrixectomy. The procedure was first described by Otto Boll in 1945. Since that time it has become the chemical of choice for chemical matrixectomies performed by podiatrists.
Concentrated liquid phenol can be used topically as a local anesthetic for otology procedures, such as myringotomy and tympanotomy tube placement, as an alternative to general anesthesia or other local anesthetics. It also has hemostatic and antiseptic qualities that make it ideal for this use.
Phenol spray, usually at 1.4% phenol as an active ingredient, is used medically to help sore throat.[33] It is the active ingredient in some oral analgesics such as Chloraseptic spray, TCP and Carmex, commonly used to temporarily treat pharyngitis.[34]
Phenol was discovered in 1834 by Friedlieb Ferdinand Runge, who extracted it (in impure form) from coal tar.[43] Runge called phenol "Karbolsäure" (coal-oil-acid, carbolic acid). Coal tar remained the primary source until the development of the petrochemical industry. In 1841, the French chemist Auguste Laurent obtained phenol in pure form.[44]
In 1836, Auguste Laurent coined the name "phène" for benzene;[45] this is the root of the word "phenol" and "phenyl". In 1843, French chemist Charles Gerhardt coined the name "phénol".[46]
The antiseptic properties of phenol were used by Sir Joseph Lister (1827–1912) in his pioneering technique of antiseptic surgery. Lister decided that the wounds themselves had to be thoroughly cleaned. He then covered the wounds with a piece of rag or lint[47]covered in phenol, or carbolic acid as he called it. The skin irritation caused by continual exposure to phenol eventually led to the introduction of aseptic (germ-free) techniques in surgery.
Joseph Lister was a student at University College London under Robert Liston, later rising to the rank of Surgeon at Glasgow Royal Infirmary. Lister experimented with cloths covered in carbolic acid after studying the works and experiments of his contemporary, Louis Pasteur in sterilizing various biological media. Lister was inspired to try to find a way to sterilize living wounds, which could not be done with the heat required by Pasteur's experiments. In examining Pasteur's research, Lister began to piece together his theory: that patients were being killed by germs. He theorized that if germs could be killed or prevented, no infection would occur. Lister reasoned that a chemical could be used to destroy the micro-organisms that cause infection.[48]
Meanwhile, in Carlisle, England, officials were experimenting with a sewage treatment, using carbolic acid to reduce the smell of sewage cess pools. Having heard of these developments and having himself previously experimented with other chemicals for antiseptic purposes without much success, Lister decided to try carbolic acid as a wound antiseptic. He had his first chance on August 12, 1865, when he received a patient: an eleven-year-old boy with a tibia bone fracture which pierced the skin of his lower leg. Ordinarily, amputation would be the only solution. However, Lister decided to try carbolic acid. After setting the bone and supporting the leg with splints, Lister soaked clean cotton towels in undiluted carbolic acid and applied them to the wound, covered with a layer of tin foil, leaving them for four days. When he checked the wound, Lister was pleasantly surprised to find no signs of infection, just redness near the edges of the wound from mild burning by the carbolic acid. Reapplying fresh bandages with diluted carbolic acid, the boy was able to walk home after about six weeks of treatment.[49]
Phenol was the main ingredient of the Carbolic Smoke Ball, an ineffective device marketed in London in the 19th century as protection against influenza and other ailments, and the subject of the famous law case Carlill v Carbolic Smoke Ball Company.
Second World War[edit]
The toxic effect of phenol on the central nervous system, discussed below, causes sudden collapse and loss of consciousness in both humans and animals; a state of cramping precedes these symptoms because of the motor activity controlled by the central nervous system.[51] Injections of phenol were used as a means of individual execution by Nazi Germany during the Second World War.[52] It was originally used by the Nazis in 1939 as part of the Aktion T4 euthanasia program.[53] The Germans learned that extermination of smaller groups was more economical by injection of each victim with phenol. Phenol injections were given to thousands of people. Maximilian Kolbe was also killed with a phenol injection after surviving two weeks of dehydration and starvation in Auschwitz when he volunteered to die in place of a stranger. Approximately one gram is sufficient to cause death.[54]
Biodegradation
Cryptanaerobacter phenolicus is a bacterium species that produces benzoate from phenol via 4-hydroxybenzoate.[60] Rhodococcus phenolicus is a bacterium species able to degrade phenol as sole carbon source.[61]
Phenols[edit]
The word phenol is also used to refer to any compound that contains a six-membered aromatic ring, bonded directly to a hydroxyl group (-OH). Thus, phenols are a class of organic compounds of which the phenol discussed in this article is the simplest member.
See also[edit]
https://en.wikipedia.org/wiki/Phenol
Antiseptics and disinfectants (D08)
Acridine derivatives
Ethacridine lactate 9-Aminoacridine Euflavine
Biguanides and amidines
Dibrompropamidine Chlorhexidine# Propamidine Hexamidine Polihexanide
Phenol and derivatives
Hexachlorophene Policresulen Phenol Triclosan Triclocarban Chloroxylenol# Biphenylol Fenticlor
Nitrofuran derivatives
Nitrofurazone
Iodine products
Iodine/octylphenoxypolyglycolether Povidone-iodine# Diiodohydroxypropane
Quinoline derivatives
Dequalinium Chlorquinaldol Oxyquinoline Clioquinol
Quaternary ammonium compounds
Benzalkonium Benzethonium chloride Cetrimonium (bromide/chloride) Cetylpyridinium Cetrimide Benzoxonium chloride Didecyldimethylammonium chloride
Mercurial products
Mercuric amidochloride Phenylmercuric borate Mercuric chloride Merbromin Nitromersol Thiomersal Mercuric iodide
Silver compounds
Silver nitrate
Alcohols
Propanol (propyl alcohol) Isopropanol (isopropyl alcohol) Ethanol (ethyl alcohol)#
Other
Potassium permanganate Sodium hypochlorite Halazone Monalazone Hydrogen peroxide Eosin Chloramine-T (tosylchloramide) Octenidine dihydrochloride
#WHO-EM ‡Withdrawn from market Clinical trials: †Phase III §Never to phase III
showvte
Throat preparations (R02)
show
Authority control Edit this at Wikidata
Categories: AntisepticsCommodity chemicalsHazardous air pollutantsOxoacidsPhenyl compounds1834 in science
https://en.wikipedia.org/wiki/Phenol
Subcategories
This category has the following 8 subcategories, out of 8 total.
A
Arsonic acids (12 P)
C
Carboxylic acids (20 C, 366 P)
H
Halogen oxoacids (15 P)
N
Nitrogen oxoacids (8 P)
P
Peroxy acids (1 C, 6 P)
Phosphorus oxoacids (8 P)
S
Sulfur oxoacids (1 C, 22 P)
T
Transition metal oxoacids (1 C, 7 P)
Pages in category "Oxoacids"
The following 11 pages are in this category, out of 11 total. This list may not reflect recent changes (learn more).
A
Arsenic acid
Arsenous acid
D
Dithionic acid
O
Orthocarbonic acid
Orthosilicic acid
Oxyacid
P
Phenol
Pyrosilicic acid
S
Selenic acid
Selenous acid
T
Tellurous acid
https://en.wikipedia.org/wiki/Category:Oxoacids
Arsenous acid (or arsenious acid) is the inorganic compound with the formula H3AsO3. It is known to occur in aqueous solutions, but it has not been isolated as a pure material, although this fact does not detract from the significance of As(OH)3.[2]
Arsonic acid is the simplest of the arsonic acids. It is a hypothetical compound, although the tautomericarsenious acid (As(OH)3) is well established. In contrast to the instability of HAsO(OH)2, the phosphorus compound with analogous stoichiometry exists as the tetrahedral tautomer. Similarly, organic derivatives such as phenylarsonic acid are tetrahedral with pentavalent central atom.[3]
There are similar acids that are the same except for having different pnictogens. The phosphorus equivalent is phosphonic acid.

https://en.wikipedia.org/wiki/Arsonic_acid
Arsonic acids are a subset of organoarsenic compounds defined as oxyacids where a pentavalent arsenic atom is bonded to two hydroxyl groups, a third oxygen atom (this one with a double bond), and an organic substituent. The salts/conjugate bases of arsonic acids are called arsonates. Like all arsenic-containing compounds, arsonic acids are toxic and carcinogenic to humans.[1][2]
Arsonic acid refers to H3AsO3, the case where the substituent is a single hydrogen atom. The other arsonic acids can simply be viewed as hydrocarbyl derivatives of this base case. Arsenic acid results when the substituent is a hydroxyl group. Methylarsonic acid results when the substituent is a methyl group. Phenylarsonic acid results when the substituent is a phenyl group.
Poultry feed[edit]
Arsanilic acid, carbarsone, nitarsone, and roxarsone were formerly used in poultry feed in order to promote growth and increase feed conversion.[3][4] In addition, nitarsone and carbarsone can also prevent histomoniasis.[5][6][7] However, concern grew over whether or not the arsenic would be ingested by humans when they ate the poultry. In 2013, a study found that chickens who were fed roxarsone and other arsenic-containing feed additives tended to show elevated levels of arsenic in breast meat—three times as high—compared to chickens that were not fed any arsenical feed additives.[8][9] On September of that year, Zoetis and Fleming Laboratories, the drugs' sponsors, voluntarily withdrew the FDA approvals for arsanilic acid, carbarsone, and roxarsone,[10] leaving only nitarsone approved until its approval for use in animal feed was withdrawn by the FDA in 2015.[11]
Medicine[edit]
Difetarsone and carbarsone can be used to treat protozoal infections and Entamoeba histolytica infections.[12][13][14][15] Difetarsonecan also be used to treat whipworm infections.[16] Arsanilic acid was discovered to treat sleeping sickness in the early 1900s,[17] but its usage in humans was discontinued after it was found to be too toxic.[18] Acetarsol is an anti-infective.[19]
https://en.wikipedia.org/wiki/Arsonic_acid_(functional_group)
Entamoeba histolytica is an anaerobic parasitic amoebozoan, part of the genusEntamoeba.[1] Predominantly infecting humans and other primates causing amoebiasis, E. histolytica is estimated to infect about 35-50 million people worldwide.[1] E. histolyticainfection is estimated to kill more than 55,000 people each year.[2] Previously, it was thought that 10% of the world population was infected, but these figures predate the recognition that at least 90% of these infections were due to a second species, E. dispar.[3] Mammals such as dogs and cats can become infected transiently, but are not thought to contribute significantly to transmission.
The word histolysis literally means disintegration and dissolution of organic tissues.
 | |
| Entamoeba histolytica trophozoite |
https://en.wikipedia.org/wiki/Entamoeba_histolytica
Histomoniasis is a commercially significant disease of poultry, particularly of chickens and turkeys, due to parasitic infection of a protozoan, Histomonas meleagridis. The protozoan is transmitted to the bird by the nematode parasite Heterakis gallinarum.[1][2] H. meleagridis resides within the eggs of H. gallinarum, so birds ingest the parasites along with contaminated soil or food.[3]Earthworms can also act as a paratenic host.
Histomonas meleagridis specifically infects the cecum and liver. Symptoms of the infection include lethargy, reduced appetite, poor growth, increased thirst, sulphur-yellow diarrhoea and dry, ruffled feathers. The head may become cyanotic (bluish in colour), hence the common name of the disease, blackhead disease; thus the name 'blackhead' is in all possibility a misnomer for discoloration.[4] The disease carries a high mortality rate, and is particularly highly fatal in poultry, and less in other birds. Currently, no prescription drug is approved to treat this disease.[3]
Poultry (especially free-ranging) and wild birds commonly harbor a number of parasitic worms with only mild health problems from them. Turkeys are much more susceptible to getting blackhead than are chickens. Thus, chickens can be infected carriers for a long time because they are not removed or medicated by their owners, and they do not die or stop eating/defecating. H. gallinarum eggs can remain infective in soil for four years, a high risk of transmitting blackhead to turkeys remains if they graze areas with chicken feces[5] in this time frame.
| Histomoniasis | |
|---|---|
| Other names | Histomonosis, blackhead disease |
 | |
| Large, pale areas in the liver of a bird infected with Histomonas meleagridis | |
| Specialty | Veterinary medicine |
https://en.wikipedia.org/wiki/Histomoniasis
Pages in category "Veterinary protozoology"
The following 30 pages are in this category, out of 30 total. This list may not reflect recent changes (learn more).
E
H
V
List[edit]
Arsenic acid is technically not an arsonic acid because the substituent is a hydroxyl group, not a hydrocarbyl group, so arsenic acid has three hydroxyl groups bound to the arsenic atom, while arsonic acids only have two.
Difetarsone is an antiprotozoal agent. Various studies have shown it to be particularly effective against Trichuris trichiura, commonly known as the whipworm. Prior to the drugs use in the early 1970s, there were few effective treatments for this infection.[1] It has also been used to treat Entamoeba histolytica infections.[2]
Difetarsone often has minor side effects, which include rashes, nausea and vomiting. It has also resulted in angioedema in at least one known case.[2]
https://en.wikipedia.org/wiki/Difetarsone
Arsanilic acid, also known as aminophenyl arsenic acid or aminophenyl arsonic acid, is an organoarsenic compound, an amino derivative of phenylarsonic acid whose amine group is in the 4-position. A crystalline powder introduced medically in the late 19th century as Atoxyl, its sodium salt was used by injection in the early 20th century as the first organic arsenical drug, but it was soon found prohibitively toxic for human use.[1]
Arsanilic acid saw long use as a veterinary feed additive promoting growth and to prevent or treat dysentery in poultry and swine.[2][3][4] In 2013, its approval by US government as an animal drug was voluntarily withdrawn by its sponsors.[5]Still sometimes used in laboratories,[6] arsanilic acid's legacy is principally through its influence on Paul Ehrlich in launching the antimicrobial chemotherapyapproach to treating infectious diseases of humans.[7]
https://en.wikipedia.org/wiki/Arsanilic_acid
Arsonic acid is the simplest of the arsonic acids. It is a hypothetical compound, although the tautomericarsenious acid (As(OH)3) is well established. In contrast to the instability of HAsO(OH)2, the phosphorus compound with analogous stoichiometry exists as the tetrahedral tautomer. Similarly, organic derivatives such as phenylarsonic acid are tetrahedral with pentavalent central atom.[3]
There are similar acids that are the same except for having different pnictogens. The phosphorus equivalent is phosphonic acid.
https://en.wikipedia.org/wiki/Arsonic_acid
Nitarsone is an organoarsenic compound that is used in poultry production as a feed additive to increase weight gain, improve feed efficiency, and prevent histomoniasis(blackhead disease).[1] It is marketed as Histostat by Zoetis.[2]
Nitarsone once was one of four arsenical food-animal drugs—along with roxarsone, arsanilic acid, and carbarsone—approved by the U.S. Food and Drug Administrationfor use in feeding poultry.[3] However, following the suspension of sales of roxarsone in the United States in 2011, nitarsone was thought to be the only arsenical animal drug actually marketed in the U.S.[3][4] In September 2013, the FDA announced that Zoetisand Fleming Laboratories agreed to voluntarily withdraw from using roxarsone, arsanilic acid, and carbarsone, which left nitarsone as the only arsenical approved in the U.S. for use in food animals.[5] But in 2015, the FDA also withdrew approval of nitarsone in animal feeds, effective at the end of 2015.[6]
https://en.wikipedia.org/wiki/Nitarsone
with the formula HOC6H4NO2. Three isomeric nitrophenols exist:
- o-Nitrophenol (2-nitrophenol; OH and NO2 groups are neighboring; CAS number: 88-75-5), a yellow crystalline solid (m.p. 46 °C).
- m-Nitrophenol (3-nitrophenol, CAS number: 554-84-7), a yellow solid (m.p. 97 °C) and precursor to the drug mesalazine (5-aminosalicylic acid).
- p-Nitrophenol (4-nitrophenol, CAS number: 100-02-7), yellow crystals (m.p. 114 °C). It is a precursor to the rice herbicide fluorodifen, the pesticide parathion, and the human analgesic paracetamol (also known as acetaminophen).
The mononitrated phenols are often hydrogenated to the corresponding aminophenolsthat are also useful industrially.[1]
https://en.wikipedia.org/wiki/Nitrophenol#Mono-nitrophenols
Roxarsone is an organoarsenic compound that has been used in poultry productionas a feed additive to increase weight gain and improve feed efficiency, and as a coccidiostat.[1] As of June 2011, it was approved for chicken feed in the United States, Canada, Australia, and 12 other countries.[2] The drug was also approved in the United States and elsewhere for use in pigs.[1][2]
Its use in the United States was voluntarily ended by the manufacturers in June 2011 and has been illegal since 2013.[3][4] Its use was immediately suspended in Malaysia.[5] It was banned in Canada in August 2011.[6] In Australia, its use in chicken feed was discontinued in 2012.[7] Roxarsone has been banned in the European Union since 1999.[8]
https://en.wikipedia.org/wiki/Roxarsone
D-amino-acid dehydrogenase (EC 1.4.99.1) is a bacterial enzyme that catalyses the oxidation of D-amino acids into their corresponding oxoacids. It contains both flavin and nonheme iron as cofactors.[1] The enzyme has a very broad specificity and can act on most D-amino acids.[2]
D-amino acid + H2O + acceptor <=> a 2-oxo acid + NH3 + reduced acceptor
This reaction is distinct from the oxidation reaction catalysed by D-amino acid oxidase that uses oxygen as a second substrate, as the dehydrogenase can use many different compounds as electron acceptors, with the physiological substrate being coenzyme Q.[1][3]
D-amino acid dehydrogenase is an enzyme that catalyzes NADPH from NADP+ and D- glucose to produce D- amino acids and glucose dehydrogenase. Some but not limited to these amino acids are D-leucine, D-isoleucine, and D-Valine, which are essential amino acids that humans cannot synthesize due to the fact that they are not included in their diet. Moreover, D- amino acids catalyzes the formation of 2-oxo acids to produce D- amino acids in the presence of DCIP which is an electron acceptor.[4] D-amino acids are used as components of pharmaceutical products, such as antibiotics, anticoagulants, and pesticides, because they have been shown to be not only more potent than their L enantiomers, but also more resistant to enzyme degradation.[5] D-amino acid dehydrogenase enzymes have been synthesized via mutagenesis with an ability to produce straight, branched, cyclic aliphatic and aromatic D-amino acids.[5] Solubilized D-amino acid dehydrogenase tends to increase its affinity for D-alanine, D-asparagine, and D--amino-n-butyrate.[6]
In E. coli K12 D-amino acid dehydrogenase is most active with D-alanine as its substrate, as this amino acid is the sole source of carbon, nitrogen, and energy. The enzyme works optimally at pH 8.9 and has a Michaelis constant for D-alanine equal to 30 mM.[7]DAD discovered in gram-negative E. coli B membrane can convert L-amino acids into D-amino acids as well.[8]
Additionally, D- amino acid dehydrogenase is used in Dye-Linked dehydrogenase (Dye-DHs) which uses artificial dyes such as 2,6-Dichloroindophenol (DCIP) as their electron acceptor rather than using their natural electron acceptors. This can accelerate the reaction between the enzyme and the substrate when the electrons are being transferred.[9]
Use in synthesis reactions
D-Amino Acid Dehydrogenase has shown itself to be effective in the synthesis of branched-chain amino acids such as D-Leucine, D-Isoleucine, and D-Valine. In the given study, researchers were successfully able to use D-amino acid dehydrogenase to create high amounts of these products from the starting material of 2-oxo acids, in the presence of ammonia. The conditions for this were variable, though the best results appeared at around 65 °C.
Amino Acids obtained through these reactions resulted in a high enantioselectivity of >99% and high yields of >99%.
Given the nature of this enzyme, it may be possible to use it in order to create non-branched D-amino acids as well as modified D-amino acids.[10]
Obtaining D-Amino Acid Dehydrogenase
In one study, in order to test the viability of using D-amino dehydrogenase in synthesis reactions, researchers used mutant bacteria to obtain and create different strains of the enzyme. These researchers found that it only required five mutations in order to modify the selective D-Amino Dehydrogenase into working with other D-amino acids. They also found that it retained its highly selective nature, capable of receiving mostly D-enantiomers after mutation, with yields in excess of 95%.[5]
A heat-stable variant of D-amino acid dehydrogenase was found in the bacterium Rhodothermus marinus JCM9785. This variant is involved in the catabolism of trans-4-hydroxy-L-proline.[11]
From the given studies, in order to obtain D-amino acid dehydrogenase one must first introduce and express it within a given bacterial species, some of which have been previously referenced. It must then be purified under favorable conditions. These are based upon the particular species of D-amino acid dehydrogenase used in a given research experiment. Under incorrect conditions, the protein may denature. For example, it was found that specifically D-alanine dehydrogenases from E. coli and P. aeruginosa would lose most of their activity when subjected to conditions of 37 - 42 °C. After this, it is possible to separate and purify through existing methods.[12]
Artificial D-Amino Acid Dehydrogenase
Due to the drawbacks of current methods, researchers have begun work on creating an artificial enzyme capable of producing the same D-amino acids as enzymes from naturally occurring sources. By adding five amino acids to a given sample isolated from U. thermosphaericus, they succeeded. By modifying the amino acid sequence, researchers were able to change the specificity of the molecule towards certain reactants and products, showing that it may be possible to use artificial D-amino acid dehydrogenase to screen for certain D-amino acid products.[13]
See also[edit]
https://en.wikipedia.org/wiki/Cold-Food_Powder
https://en.wikipedia.org/wiki/Cilobamine
https://en.wikipedia.org/wiki/1-(3-Chlorophenyl)-4-(2-phenylethyl)piperazine
https://en.wikipedia.org/wiki/Camfetamine
https://en.wikipedia.org/wiki/Azabon
https://en.wikipedia.org/wiki/Cathinone
https://en.wikipedia.org/wiki/Benzphetamine
https://en.wikipedia.org/wiki/Hydroxybupropion
https://en.wikipedia.org/wiki/Hexacyclonate
https://en.wikipedia.org/wiki/Bupropion
https://en.wikipedia.org/wiki/Bemegride
https://en.wikipedia.org/wiki/GTS-21
https://en.wikipedia.org/wiki/G-130
https://en.wikipedia.org/wiki/FP-β-CPPIT
https://en.wikipedia.org/wiki/Flurothyl
https://en.wikipedia.org/wiki/AR-R17779
https://en.wikipedia.org/wiki/2-Amino-1,2-dihydronaphthalene
https://en.wikipedia.org/wiki/(%2B)-CPCA
https://en.wikipedia.org/wiki/Pethidine
https://en.wikipedia.org/wiki/LR-5182
https://en.wikipedia.org/wiki/Cypenamine
https://en.wikipedia.org/wiki/Cyclopentamine











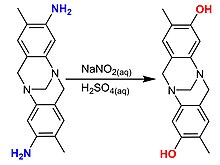

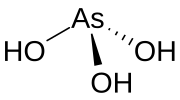


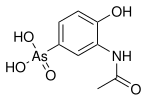

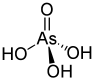
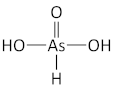









No comments:
Post a Comment